
AMOROLFINE BLUEFISH 50 mg/ml MEDICINAL NAIL LACQUER

How to use AMOROLFINE BLUEFISH 50 mg/ml MEDICINAL NAIL LACQUER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Amorolfina Bluefish 50 mg/ml medicinal nail varnish
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
Use this medication exactly as described in this package leaflet or as your doctor or pharmacist has indicated.
- Keep this package leaflet, as you may need to read it again.
If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- Consult your doctor if you feel worse after three months.
Contents of the Package Leaflet
- What is Amorolfina Bluefish and what is it used for
- What you need to know before taking Amorolfina Bluefish
- How to take Amorolfina Bluefish
- Possible side effects
- Storage of Amorolfina Bluefish
- Package Contents and Additional Information
1. What is Amorolfina Bluefish and what is it used for
Amorolfina Bluefish contains amorolfina (as hydrochloride) as the active ingredient, which belongs to the group of medications known as antifungals. It eliminates a wide variety of fungi that can cause nail infections. Amorolfina Bluefish is used to treat fungal nail infections.
2. What you need to know before taking Amorolfina Bluefish
Do not use Amorolfina Bluefish
- If you are allergic to amorolfina or any of the other components of this medication (listed in section 6).
- If you are under 18 years old.
This medication may cause allergic reactions, some of which can be severe. If this occurs, discontinue treatment, immediately remove the medication with nail polish remover or the alcohol wipes included in the package, and seek medical advice. The medication should not be reapplied. If you experience any of the following symptoms, you should seek urgent medical attention if you have any of the following symptoms:
- Difficulty breathing.
- Your face, lips, tongue, or throat are swollen.
- If your skin has developed a severe rash.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Amorolfina Bluefish.
After applying Amorolfina Bluefish, a minimum interval of 10 minutes should be respected before applying any cosmetic nail polish. Before reapplying Amorolfina Bluefish, the cosmetic nail polish should be carefully removed.
Taking into account the status of the medication (non-prescription medication to be dispensed exclusively in pharmacies), the medication should not be dispensed in the situations described below, unless medically indicated:
Special Recommendations
- Avoid contact between the varnish and the eyes, ears, and mucous membranes.
- Amorolfina Bluefish is not recommended for children under 18 years old.
- Healthy nails should not be filed with the same file used for infected nails.
- Nail polish or artificial nails should not be used during treatment with Amorolfina Bluefish.
- Patients working with organic solvents (diluents, turpentine, etc.) should wear impermeable gloves to protect Amorolfina Bluefish on the nails.
- Amorolfina Bluefish should not be applied to the skin around the nail. There is a possibility that a local or systemic allergic reaction may occur after using this medication. If this happens, the medication should be discontinued immediately and medical attention sought. The medication should be removed with a nail polish remover. The medication should not be reapplied.
Other Medications and Amorolfina Bluefish
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
There are no studies on the interaction of Amorolfina Bluefish with other medications. Inform your doctor or pharmacist if you notice any side effects.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication.
Experience with the use of amorolfina during pregnancy and breastfeeding is limited. Amorolfina Bluefish should not be used during pregnancy and breastfeeding unless clearly necessary.
This medication contains alcohol (ethanol). Ethanol is flammable, and Amorolfina Bluefish should not be used near an open flame, lit cigarettes, or certain appliances (e.g., hair dryers).
3. How to Take Amorolfina Bluefish
Follow your doctor's instructions for taking this medication exactly. If in doubt, consult your doctor or pharmacist again.
Before Starting Treatment:
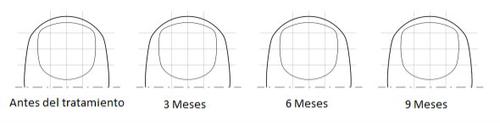
In the following diagram, shade the area affected by the fungal infection on the nail. This will help you remember what the nail looked like originally when your treatment is reviewed. Every three months, shade the affected area until the infected nail has grown out completely. If you have more than one nail affected, select the most affected nail for this exercise. Bring this package leaflet to your doctor or pharmacist when the treatment is reviewed to inform them about the progress of the treatment to date.
Amorolfina Bluefish should be applied to the affected fingernails, one or two times a week, or to the toenails once a week, exactly as your doctor indicates (normally, one weekly application is sufficient).
- File the nail
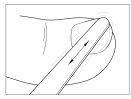 File the affected parts of the nail as deeply as possible, including the surface, using one of the files provided in the package.
File the affected parts of the nail as deeply as possible, including the surface, using one of the files provided in the package.
WARNING: Healthy nails should never be filed with the file used for infected nails, as this can spread the infection. To prevent the spread of infection, avoid letting someone else use the files from your package.
- Clean the nail
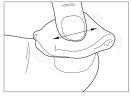
Use one of the wipes provided to clean the surface of the nail. Save the compress or cotton ball to clean the spatula later. Repeat steps 1 and 2 for each affected nail.
- Take some varnish from the bottle

Dip one of the reusable spatulas into the nail varnish bottle. Do not clean the spatula on the edge of the bottle before application.
- Apply the varnish
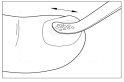 Apply the varnish evenly over the entire surface of the nail. Clean the reusable spatula between each pass from one nail to another to avoid contaminating the varnish. Repeat steps 3 and 4 for each affected nail. Close the bottle immediately after each application.
Apply the varnish evenly over the entire surface of the nail. Clean the reusable spatula between each pass from one nail to another to avoid contaminating the varnish. Repeat steps 3 and 4 for each affected nail. Close the bottle immediately after each application.
- Let it dry
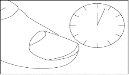
Let the treated nail(s) dry for approximately 3 minutes.
- Clean the spatula
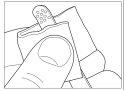 The spatulas provided are reusable. However, it is essential to clean them as thoroughly as possible after completing each treatment, using the same wipe used for nail cleaning. Avoid touching the treated nails with the wipe. Close the varnish bottle tightly. Dispose of the wipe carefully, as it is flammable.
The spatulas provided are reusable. However, it is essential to clean them as thoroughly as possible after completing each treatment, using the same wipe used for nail cleaning. Avoid touching the treated nails with the wipe. Close the varnish bottle tightly. Dispose of the wipe carefully, as it is flammable.
Before reusing Amorolfina Bluefish, first remove any remaining varnish from the nails using a wipe, then refile the nails if necessary. Reapply the varnish as described above.
Once dry, the nail varnish will not be affected by soap or water, so you can wash your hands and feet as usual. If you need to use chemicals, such as paint thinners or turpentine, you should wear rubber or impermeable gloves to protect the nail varnish on your hands.
Average Treatment Duration
Treatment should be continued without interruption until a healthy nail has grown out completely and the affected areas have healed. The duration of treatment depends essentially on the intensity, location of the infection, and the growth capacity of the nails. Generally, this translates to 6 months for fingernails and 9 to 12 months for toenails.
If there is no improvement after 3 months of treatment, you should consult your doctor.
Important
- Wait until the medicinal varnish has dried before washing your hands or feet.
- Do not use artificial nails during treatment with Amorolfina Bluefish.
- If Amorolfina Bluefish gets into your eyes or ears, rinse them immediately with water and contact your doctor, pharmacist, or the nearest hospital.
- Avoid contact between the varnish and mucous membranes (e.g., mouth and nostrils). Do not inhale it.
- Use impermeable gloves when using solvents or diluents (e.g., turpentine) to avoid removing the medicinal varnish.
- Do not apply Amorolfina Bluefish to the skin around the nail. This product contains ethanol (alcohol). Incorrect use of Amorolfina Bluefish can cause skin irritation or dryness around the nail.
- Do not inhale the medicinal varnish.
- If you or anyone else accidentally swallows the varnish, contact your doctor, pharmacist, or the nearest hospital immediately.
- Cosmetic nail polishes based on nitrocellulose (cellulose nitrate) can be applied (check the ingredient list; most cosmetic nail polishes contain nitrocellulose). You should wait at least 10 minutes after applying Amorolfina Bluefish before painting your nails. Before reapplying Amorolfina Bluefish, the cosmetic nail polish should be carefully removed. Until a new application of Amorolfina Bluefish is made, the use of cosmetic nail polish removers should be avoided.
If You Stop Using Amorolfina Bluefish
It is essential to continue using Amorolfina Bluefish until the infection has disappeared and healthy nails have grown out. This usually takes 6 months for fingernails and 9 to 12 months for toenails. Your doctor will likely check on the progress of your treatment every 3 months.
If you have any questions about using this product, consult your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The side effects of this medication are rare or very rare.
Rare side effects(may affect up to 1 in 1,000 people): damaged, discolored, fragile, or brittle nails.
Very rare side effects(may affect up to 1 in 10,000 people): a burning sensation may occur in the area around the nails.
Frequency not known(cannot be calculated from available data): Systemic allergic reaction (severe allergic reaction that may be associated with swelling of the face, lips, tongue, or throat, difficulty breathing, and/or severe skin rash).
Redness of the skin, itching, contact dermatitis around the nail, hives, or blisters.
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Amorolfina Bluefish
Keep this medication out of the sight and reach of children.
Store below 30°C.
Protect from heat. Keep the bottle tightly closed and in a vertical position.
Do not use this medication after the expiration date, which appears on the packaging and bottle after CAD. The expiration date is the last day of the month indicated.
Do not use this medication if you notice visible signs of deterioration.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Amorolfina Bluefish
- The active ingredient is amorolfina as hydrochloride.
- The other ingredients are: ammonio methacrylate copolymer (type A), triacetin, n-butyl acetate, ethyl acetate, anhydrous ethanol.
Appearance of Amorolfina Bluefish and Package Contents
Amorolfina Bluefish is a clear, colorless to pale yellow solution.
The medicinal nail varnish, Amorolfina Bluefish, is available in 2.5 ml or 5 ml amber glass bottles.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Bluefish Pharmaceuticals AB
P.O. Box 49013
100 28 Stockholm
Sweden
Manufacturer:
Chanelle Medical Unlimited Company
Loughrea,
County Galway, Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Bluefish Pharma S.L.U.,
AP 36007
2832094 Madrid, Branch 36
This medication is authorized in the Member States of the European Economic Area under the following names:
Member State | Medication Name |
Portugal | Amorolfine Bluefish 5% medicinal nail varnish |
Spain | Amorolfina Bluefish 50mg/ml medicinal nail varnish |
Italy | Amorolfina Bluefish 5% medicinal nail polish |
Date of the Last Revision of this Package Leaflet: June 2024
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price21.43 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMOROLFINE BLUEFISH 50 mg/ml MEDICINAL NAIL LACQUERDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Isdin S.A.Prescription requiredDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Laboratorio Stada S.L.Prescription requiredDosage form: NAIL POLISH, 50mg/ml Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Teva Pharma S.L.U.Prescription required
Online doctors for AMOROLFINE BLUEFISH 50 mg/ml MEDICINAL NAIL LACQUER
Discuss questions about AMOROLFINE BLUEFISH 50 mg/ml MEDICINAL NAIL LACQUER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








