

Formoterol Easihaler

Ask a doctor about a prescription for Formoterol Easihaler

How to use Formoterol Easihaler
1. What is Formoterol Easyhaler and what is it used for
What is Formoterol Easyhaler?
Formoterol Easyhaler is a medicine used to treat asthma, which relaxes the airways and thus prevents and treats symptoms such as wheezing, shortness of breath, and coughing.
The active substance is formoterol. Formoterol in powder form is in an inhaler called Easyhaler. The powder is inhaled into the lungs through the inhaler mouthpiece.
What is Formoterol Easyhaler used for?
It is used:
- in combination with inhaled corticosteroids to treat and prevent asthma symptoms,
- to treat and prevent symptoms of chronic obstructive pulmonary disease (COPD).
The effect of formoterol starts within 1-3 minutes and lasts for about 12 hours.
2. Important information before using Formoterol Easyhaler
You should not use Formoterol Easyhaler if you are allergic to:
formoterol,
any other ingredient of this medicine (listed in section 6), which is lactose monohydrate (which contains a small amount of milk proteins).
You should not give it to children under 6 years of age.
Warnings and precautions
Before starting to use Formoterol Easyhaler, you should discuss it with your doctor, pharmacist, or nurse if you have:
heart problems,
high blood pressure,
diabetes (you may need to have your blood glucose levels checked more often when you start using Formoterol Easyhaler),
low potassium levels in your blood,
overactive thyroid gland,
phaeochromocytoma.
Formoterol Easyhaler with other medicines
You should tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to take.
You should inform your doctor, especially if you are taking medicines such as:
- monoamine oxidase inhibitors (MAOIs), such as moclobemide used to treat depression. You should not use Formoterol Easyhaler if you have been treated with MAOIs in the last 14 days.
- tricyclic antidepressants, such as amitriptyline or imipramine.
- medicines used to treat Parkinson's disease, such as selegiline and levodopa.
- medicines used to treat heart conditions, including irregular heartbeat or angina.
- medicines used to treat high blood pressure.
- beta-blockers (in tablet and eye drop form).
- diuretics.
- erythromycin (used to treat infections).
- corticosteroids in tablet form (such as prednisolone).
- medicines used to treat respiratory disorders, such as theophylline or aminophylline.
- medicines used to treat allergies, such as antihistamines.
- medicines used to treat mental illness or severe nausea and vomiting, such as phenothiazines.
- medicines used to treat thyroid disorders, such as levothyroxine.
If you are going to have an operation, you should tell your doctor or dentist that you are using Formoterol Easyhaler.
Formoterol Easyhaler with food, drink, and alcohol
If you drink alcohol while using Formoterol Easyhaler, you may experience a rapid heart rate.
Pregnancy, breastfeeding, and fertility
You should not use Formoterol Easyhaler if you are pregnant or think you may be pregnant or if you are planning to become pregnant, unless your doctor advises you to do so. You should not use Formoterol Easyhaler if you are breastfeeding.
Driving and using machines
Formoterol is unlikely to affect your ability to drive or use machines. However, if you experience side effects such as dizziness, your ability to drive or use machines may be impaired.
Formoterol Easyhaler contains lactose monohydrate
Lactose monohydrate contains small amounts of milk proteins, which may cause an allergic reaction.
If you have previously been diagnosed with an intolerance to some sugars, you should contact your doctor before taking this medicine. The amount of lactose monohydrate in Formoterol Easyhaler (about 8 mg per dose) is unlikely to cause problems in people with lactose intolerance.
3. How to use Formoterol Easyhaler
This medicine should always be used as directed by your doctor or pharmacist. If you are unsure, you should ask your doctor or pharmacist.
Asthma attacks
If you experience an asthma attack between doses of Formoterol Easyhaler, you should use a short-acting inhaler as needed.
You should contact your doctor as soon as possible if:
- your wheezing, shortness of breath, or coughing gets worse,
- your symptoms come back sooner than usual,
- you need to use your short-acting inhaler more often than usual.
Use in adults (including the elderly) and adolescents (12 years and older):
Asthma:
Usually 1 dose (12 micrograms) taken regularly twice a day. The dose can be increased to a maximum of 2 doses (2 x 12 micrograms) per day.
Chronic obstructive pulmonary disease (COPD):
Usually 1 dose (12 micrograms) taken regularly twice a day. The maximum daily dose is 2 doses per day.
Use in children aged 6 to 12 years:
Asthma:
Usually 1 dose (12 micrograms) taken regularly twice a day. The maximum daily dose is 2 inhalations per day.
Using a higher dose of Formoterol Easyhaler than recommended
If you have taken more Formoterol Easyhaler than you should, you should contact your doctor or nurse. Symptoms of overdose may include: nausea, vomiting, headache, tremors, and rapid heart rate.
Missing a dose of Formoterol Easyhaler
You should not take a double dose to make up for a missed dose. If you miss a dose, you should take the missed dose as soon as possible. If it is almost time for your next dose, do not take the missed dose, but continue with your regular dosing schedule.
Stopping use of Formoterol Easyhaler
You should not stop or reduce the dose of Formoterol Easyhaler, inhaled corticosteroids, or other respiratory medicines without consulting your doctor. It is important to take your medicine regularly, even if you feel better. This medicine is usually taken in the morning and evening.
If you have any further questions about using this medicine, you should ask your doctor, pharmacist, or nurse.
Instructions for using the Easyhaler inhaler are at the end of the leaflet.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. These side effects are usually mild and may disappear during continued treatment. If side effects are severe, persist for more than a few days, or if you are concerned about any of them, you should tell your doctor.
You should stop using Formoterol Easyhaler and contact your doctor immediately if you experience any of the following symptoms:
- itching, rash, skin redness,
- swelling of the eyelids, lips, face, or throat,
- low blood pressure or collapse,
- worsening of wheezing and shortness of breath immediately after taking a dose of Formoterol Easyhaler.
Other side effects:
Common ( may affect up to 1 in 10 people):
- tremors,
- palpitations,
- headache.
Uncommon ( may affect up to 1 in 100 people):
- muscle cramps, muscle pain,
- rapid heart rate,
- insomnia,
- nervousness, anxiety.
Rare ( may affect up to 1 in 1000 people):
- irregular or uneven heartbeat, extra heartbeats,
- low potassium levels in the blood,
- irritation of the mouth or throat,
- wheezing, shortness of breath,
- allergic reactions,
- nausea (vomiting).
Very rare ( may affect up to 1 in 10,000 people):
- taste disturbances,
- increased blood glucose levels,
- chest pain or tightness,
- heart problems (prolonged QT interval),
- changes in blood pressure,
- dizziness.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Department of Drug Safety, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181 C, 02-222 Warszawa, Tel.: +48 22 49 21 301, Fax: + 48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl
You can also report side effects to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Formoterol Easyhaler
- The medicine should be stored out of the reach and sight of children.
- Before first use, store in a closed foil bag.
- After opening the foil bag: do not store above 30 ° C, protect from moisture. It is recommended to store the Easyhaler inhaler in its protective packaging.
- If the Formoterol Easyhaler gets wet, it should be replaced with a new one.
- Formoterol Easyhaler should be replaced with a new one no later than 4 months after opening the foil bag. To remember the opening date, you should write it down.
Do not use Formoterol Easyhaler after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Formoterol Easyhaler contains
- The active substance is formoterol fumarate dihydrate.
- The other ingredients are lactose monohydrate (which contains a small amount of milk proteins).
What Formoterol Easyhaler looks like and contents of the pack
White to off-white powder in a plastic multi-dose inhaler. Each inhaler contains 120 doses and has a white body with a green upper part. The inhaler is packed in a foil bag and may be packaged with or without protective packaging in a cardboard box. Formoterol Easyhaler 12 micrograms/dose, powder for inhalation may be available in packs of 1 or 2 inhalers.
- one inhaler (120 doses) + protective packaging for the inhaler
- one inhaler (120 doses)
- two inhalers (2 x 120 doses)
Not all pack sizes may be marketed.
Marketing authorization holder
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
Manufacturer
Orion Corporation
Orionintie 1
02200 Espoo
Finland
To obtain more detailed information on this medicine, you should contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o. o.
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Czech Republic, Finland, Slovakia, Poland, Hungary, United Kingdom
Formoterol Easyhaler
Denmark
Formo Easyhaler
Estonia, Lithuania, Latvia
Fomeda Easyhaler
Date of last revision of the leaflet:12.03.2021
Detailed and up-to-date information on the use of this medicine is available after scanning the QR code on the outer packaging and the inhaler label. The same information is available on the website: www.oeh.fi/fpl
QR code for the website: www.oeh.fi/fpl
How to use the Easyhaler inhaler
Information about the Easyhaler inhaler
The Formoterol Easyhaler inhaler may be different from the inhalers you have used before. Therefore, it is very important to use it correctly, as incorrect use may result in you not receiving the correct dose of medicine. This may lead to your condition getting worse or your asthma or COPD not being treated properly.
Your doctor, nurse, or pharmacist will show you how to use the inhaler correctly. You should make sure you understand how to use the inhaler correctly. If you have any doubts, you should contact your doctor, nurse, or pharmacist. As with all inhalers, carers should ensure that children who have been prescribed Formoterol Easyhaler use the correct inhalation technique, as described below. You can also use the video instructions at: www.oeh.fi/fpl
Using the Easyhaler inhaler for the first time
| The Easyhaler inhaler is supplied in a foil bag. Do not open the bag until you are ready to start using the medicine, as it helps keep the powder in the inhaler dry. If you are ready to start treatment, open the packaging and write down the opening date, e.g. in a calendar. The Formoterol Easyhaler inhaler can be used for 4 months after removal from the foil bag. | 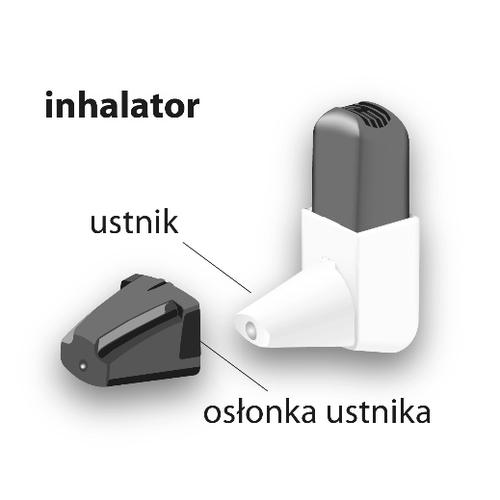 |
HOW TO USE THE INHALER
Step 1: SHAKING
| SHAKE 3 TO 5 TIMES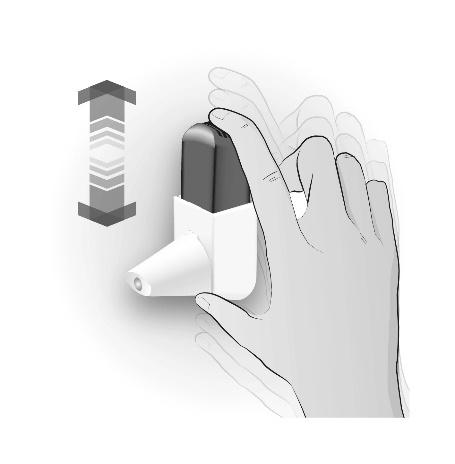 | Important information to remember
|
Step 2: CLICKING
| CLICK ONCE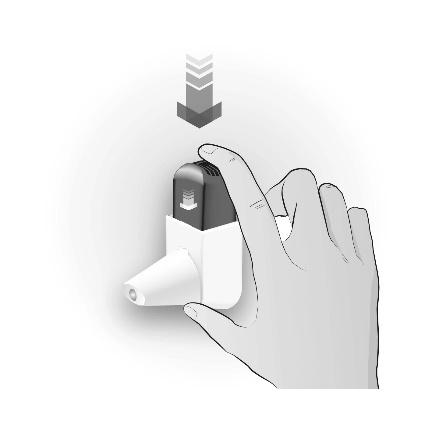 | Important information to remember
|
If you need to take an additional inhalation, you should repeat the steps described in points 1-3 "Shaking, Clicking, Inhaling".
After using the inhaler:
- Replace the mouthpiece cover to prevent accidental activation of the inhaler.
Step 3: INHALATION
| INHALATION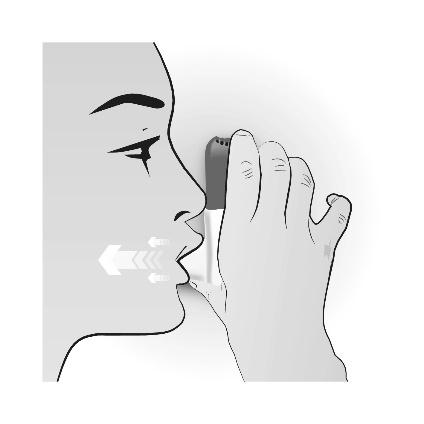 | Important information to remember
|
How to remove powder from the mouthpiece If you accidentally click the inhaler or click it multiple times, or breathe out into the inhaler, you should remove the powder from the mouthpiece:
| 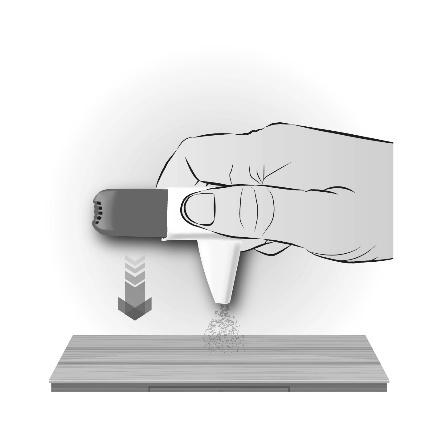 |
Cleaning the Easyhaler inhaler
You should keep the inhaler dry and clean. If necessary, the mouthpiece of the inhaler can be cleaned with a dry cloth or tissue. Do not use water. The powder in the Easyhaler inhaler is sensitive to moisture.
| Using the Easyhaler inhaler in its protective packaging The inhaler can be used in its protective packaging, which helps to extend the product's shelf life. Before placing the inhaler in the protective packaging, you should make sure that the mouthpiece cover is covering the mouthpiece, which prevents accidental activation of the inhaler. You can use the inhaler without removing it from the protective packaging. Use as described above, |
| 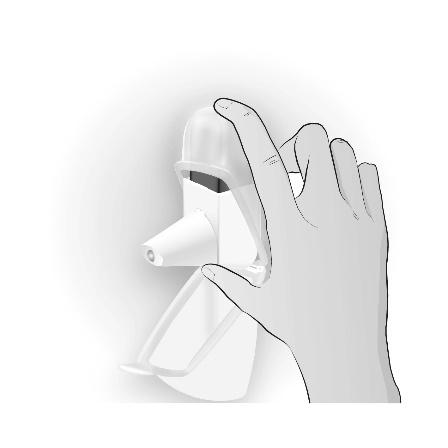 |
| Switching to a new Easyhaler inhaler The inhaler has a dose counter, which shows the number of doses left. The counter turns every five activations. When the dose counter turns red, it means that there are 20 doses left. If you do not have a new Easyhaler inhaler yet, you should contact your doctor to get a new prescription. When the dose counter shows 0, you should replace the Easyhaler inhaler. If you are using the protective packaging, you should keep it and place the new inhaler in it. | 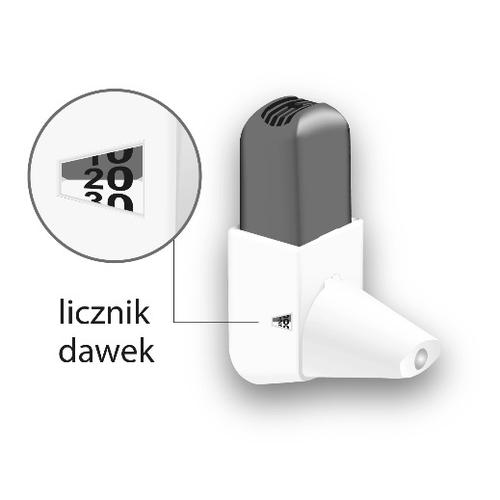 |
To remember
- 1. Shaking, 2. Clicking, 3. Inhaling.
- Do not allow the inhaler to get wet, keep it away from moisture .
If you have any further questions about using this medicine, you should ask your doctor or pharmacist.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOrion Corporation
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Formoterol EasihalerDosage form: Aerosol, 12 mcg/measured doseActive substance: formoterolManufacturer: Chiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbHPrescription requiredDosage form: Powder, 12 mcgActive substance: formoterolPrescription requiredDosage form: Powder, 12 mcg/inh. doseActive substance: formoterolPrescription required
Alternatives to Formoterol Easihaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Formoterol Easihaler in Ukraina
Alternative to Formoterol Easihaler in Hiszpania
Online doctors for Formoterol Easihaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Formoterol Easihaler – subject to medical assessment and local rules.














