
Foradil

Ask a doctor about a prescription for Foradil

How to use Foradil
Leaflet accompanying the packaging: patient information
Foradil, 12 micrograms, powder for inhalation in hard capsules
Formoterol fumarate dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Foradil and what is it used for
- 2. Important information before using Foradil
- 3. How to use Foradil
- 4. Possible side effects
- 5. How to store Foradil
- 6. Contents of the pack and other information
1. What is Foradil and what is it used for
The active substance of Foradil is formoterol fumarate dihydrate. It is a bronchodilator. Its action is based on the relaxation of smooth muscles in the bronchi, which makes breathing easier. This effect occurs quickly (within 1-3 minutes) and lasts for 12 hours after inhalation. Each hard capsule contains 12 micrograms of formoterol fumarate dihydrate and is intended for use with the Aerolizer inhaler.
Foradil is indicated for:
- prevention and treatment of bronchial constriction in patients with asthma, as an adjunct to inhaled glucocorticosteroids,
- prevention of bronchial constriction induced by allergens, cold air, or physical exertion,
- prevention and treatment of bronchial constriction in patients with reversible or irreversible chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. Foradil improves the quality of life in patients with COPD.
The bronchodilatory effect lasts for 12 hours after Foradil inhalation. Therefore, maintenance treatment, which involves using the medicine twice a day, usually leads to the relief of bronchial constriction associated with chronic conditions, both during the day and at night.
2. Important information before using Foradil
When not to use Foradil
- if the patient is allergic to formoterol fumarate dihydrate, lactose (which contains a small amount of milk proteins), or any of the other ingredients of Foradil (excipients listed in section 6, at the end of the leaflet).
If this applies to the patient, they should not take Foradil and should consult their doctor. If the patient thinks they may be allergic, they should discuss this with their doctor.
Warnings and precautions
Before starting treatment with Foradil, the patient should discuss the following with their doctor:
- if the patient has heart disease, including arrhythmias;
- if the patient has high blood pressure;
- if the patient has diabetes;
- if the patient has hyperthyroidism;
- if the patient has an aneurysm (a bulge in the wall of an artery caused by its weakening);
- if the patient has a heart condition related to an abnormal electrical impulse, known as "QT interval prolongation";
- if the patient has a pheochromocytoma (a tumor of the adrenal gland that can affect blood pressure);
- if the patient has tachycardia;
- if the patient has severe heart failure. The patient should immediately inform their doctor or pharmacist if any of these symptoms occur during treatment with Foradil.
Important information Do not swallow the capsules - the capsules should be used by inhaling their contents through the Aerolizer inhaler.
Aerolizer. If the doctor has prescribed regular use of other medicines for respiratory diseases, it is essential to continue taking them regularly. DO NOT STOPtaking the medicine or reduce its dose without consulting a doctor, even if there has been a significant improvement in the patient's condition. If the patient experiences shortness of breath or wheezing while using Foradil, they should continue the treatment and contact their doctor as soon as possible, as a change in treatment may be necessary. In patients with diabetes, the doctor may recommend monitoring blood glucose levels. In patients with asthma, Foradil should not be used as the only medicine controlling asthma. Foradil should always be used in combination with an inhaled corticosteroid. While using Foradil, the patient should not use other products containing long-acting beta2-adrenergic receptor agonists, such as salmeterol.
Do not use Foradil if:
- the patient's condition is well-controlled with an inhaled corticosteroid.
- the patient only needs a short-acting beta2-adrenergic receptor agonist occasionally.
In clinical trials with Foradil, severe asthma attacks (see section 4 "Possible side effects") have been observed. The patient should not start using Foradil or increase the dose prescribed by their doctor during an asthma attack. The patient should not change or stop taking any medicines for controlling or treating breathing problems, including inhaled corticosteroids. In the case of asthma, Foradil should not be used to relieve sudden wheezing. To treat sudden asthma symptoms, the patient should always use a short-acting beta2-adrenergic receptor agonist (a rescue inhaler prescribed by their doctor).
Important information about a similar medicine
Foradil belongs to a group of medicines called long-acting beta2-adrenergic receptor agonists. A large study with another long-acting beta2-adrenergic receptor agonist showed an increased risk of asthma-related death. It has not been determined whether Foradil has a similar effect. The patient should discuss the benefits and risks of asthma treatment with Foradil with their doctor.
Monitoring during treatment with Foradil
Treatment with Foradil may cause an increase in blood glucose levels. Therefore, in patients with diabetes, it may be necessary to monitor blood glucose levels. Treatment with Foradil may cause a decrease in blood potassium levels, increasing the patient's susceptibility to heart rhythm disorders. Therefore, especially in the case of severe asthma, the doctor may recommend monitoring blood potassium levels. If the patient has any questions about how Foradil works or why it has been prescribed, they should consult their doctor.
Paradoxical bronchospasm
As with other inhaled medicines, Foradil may cause paradoxical bronchospasm. In such cases, the patient should stop using the medicine immediately and contact their doctor, who may prescribe alternative treatment.
Children and adolescents
Foradil is not recommended for use in children under 6 years of age.
Use in elderly patients (over 65 years of age)
In elderly patients, the same dose of Foradil can be used as in adult patients.
Foradil and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. It may be necessary to change the dosage or, in some cases, stop taking one of the medicines. This applies to both prescription and over-the-counter medicines, especially:
- monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants (medicines used to treat depression and mood changes);
- sympathomimetic medicines (medicines similar to adrenaline used to treat asthma or reduce nasal congestion);
- antihistamines (medicines used to prevent or treat allergic reaction symptoms);
- steroids (medicines often used to treat asthma and other inflammatory diseases);
- diuretics (medicines used to treat swelling caused by fluid retention, heart failure, and high blood pressure);
- beta-adrenergic receptor blockers (medicines used to treat high blood pressure, heart failure, angina, anxiety, and heart rhythm disorders. Some eye drops used to treat glaucoma may contain beta-adrenergic receptor blockers);
- quinidine, disopyramide, procainamide (medicines used to treat heart rhythm disorders);
- phenothiazine derivatives (medicines used to treat mental disorders, such as schizophrenia, manic excitement, psychotic reactions, and anxiety);
- digitalis glycosides (medicines used to treat heart failure and heart rhythm disorders);
- xanthine derivatives (medicines used to treat asthma or other chronic obstructive pulmonary diseases);
- macrolides (e.g., erythromycin, azithromycin) used to treat bacterial infections;
- general anesthetics used during surgical procedures, such as halogenated hydrocarbons (e.g., halothane). The patient should inform their doctor about using Foradil if they are planning to undergo surgery under general anesthesia;
- anticholinergic medicines (e.g., ipratropium bromide) used to treat gastrointestinal diseases, urinary tract disorders, etc.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Pregnancy Foradil should not be used during pregnancy unless clearly prescribed by a doctor. The doctor will inform the patient about the potential risks associated with using Foradil during pregnancy. Breastfeeding If the patient is breastfeeding, they should consult their doctor before using Foradil.
Driving and using machines
If the patient experiences dizziness or other similar side effects, they should not drive or operate machinery.
Foradil contains lactose
If the patient has a severe lactose intolerance, they should inform their doctor before using Foradil. The excipient lactose contains a small amount of milk proteins, which may cause allergic reactions.
3. How to use Foradil
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist. The patient should not exceed the recommended dose.
Do not swallow the capsules - the capsules should be used by inhaling their contents through the Aerolizer inhaler.
Dosage
Adults
Asthma
In the treatment of asthma, Foradil should always be prescribed as an add-on therapy to be used in combination with an inhaled corticosteroid. Maintenance treatment: inhalation of the contents of 1 to 2 capsules for inhalation (12 to 24 micrograms) twice a day. The maximum recommended maintenance dose is 4 capsules (48 micrograms) per day. If necessary, the doctor may prescribe an additional 1 to 2 capsules per day to reduce the severity of symptoms, provided that the maximum recommended daily dose of 48 micrograms is not exceeded. If the need for additional doses occurs frequently (e.g., more than two days a week), the patient should inform their doctor as soon as possible, as this may indicate worsening asthma. In the case of a sudden asthma attack, the patient should use a short-acting bronchodilator (a rescue inhaler prescribed by their doctor).
Prevention of bronchial constriction induced by physical exertion or allergens
Inhalation of the contents of one capsule for inhalation (12 micrograms) at least 15 minutes before physical exertion or exposure to an allergen. In patients with severe bronchial constriction, it may be necessary to use 2 capsules for inhalation (24 micrograms). In the treatment of asthma, inhaled corticosteroids are always used.
Chronic obstructive pulmonary disease
Maintenance treatment: inhalation of the contents of 1 to 2 capsules for inhalation (12 to 24 micrograms) twice a day.
Use in children over 6 years of age and adolescents
Asthma:
Maintenance treatment: inhalation of the contents of one capsule for inhalation (12 micrograms) twice a day. The maximum recommended daily dose is 2 capsules. In the case of a sudden asthma attack, the patient should use a short-acting bronchodilator (e.g., salbutamol).
Prevention of bronchial constriction induced by physical exertion or allergens
Inhalation of the contents of one capsule for inhalation (12 micrograms) at least 15 minutes before physical exertion or exposure to an allergen. Children over 6 years of age may use Foradil only if they can properly use the inhaler (see "Inhaler instructions") and only with the help of an adult. Foradil is not recommended for use in children under 6 years of age.
Inhaler instructions
Immediately before use, the capsule should be removed from the blister pack. The patient should ensure that their hands are completely dry to avoid moistening the capsule. The patient should not swallow the capsule. The powder in the capsule is intended for inhalation only.
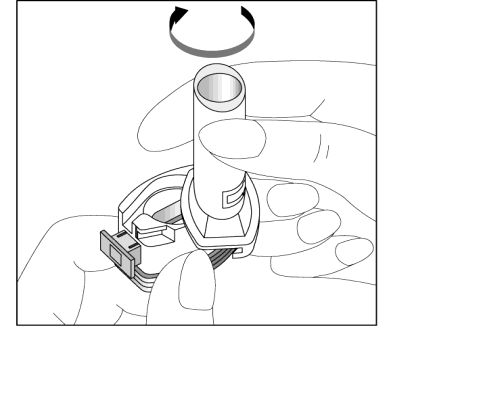
- 1. Remove the cap.
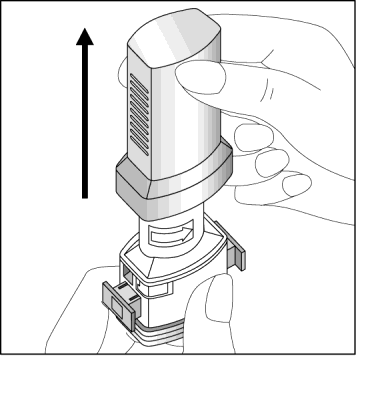
- 2. Hold the base of the inhaler firmly and open it by twisting the mouthpiece in the direction indicated by the arrow.
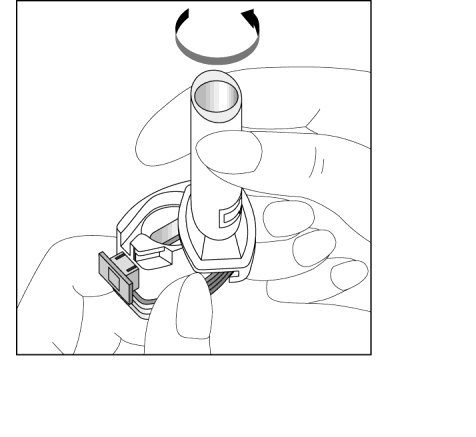
- 3. Place the capsule in the capsule-shaped compartment in the base of the inhaler. The capsule should be removed from the blister pack immediately before use.
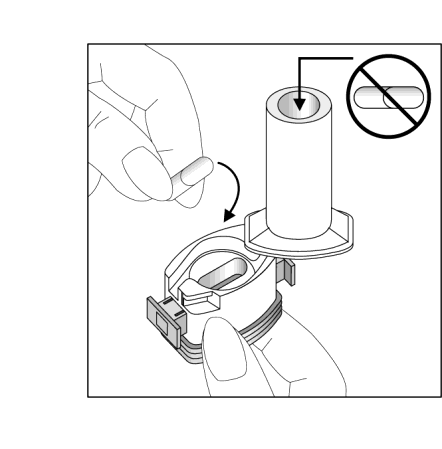
- 4. Twist the mouthpiece to the closed position.
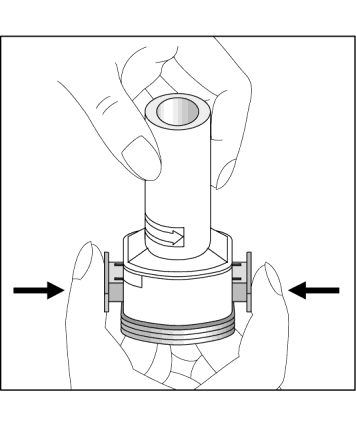
- 5. Press the blue buttons once, holding the inhaler in a vertical position.
Release the buttons. Note: At this point, the capsule may break, and small pieces of gelatin may enter the mouth or throat. Since gelatin is edible, its consumption is not harmful. The likelihood of such an event is minimal if the capsule is not pierced more than once, storage conditions are maintained, and the capsule is removed from the blister pack immediately before use (see point 3).
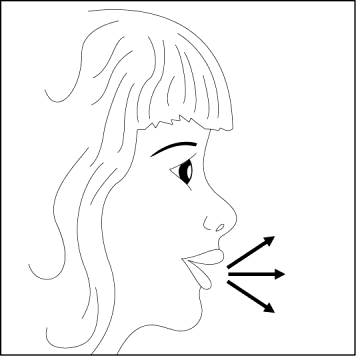
- 6. Exhale deeply.
- 7. Place the mouthpiece in the mouth and tilt the head slightly backwards. Close the lips around the mouthpiece and take a quick, but measured and deep breath. During this time, the capsule should rotate in the inhaler chamber, and the powder should be dispersed, producing a characteristic sound. If this sound does not occur, it may indicate that the capsule is stuck in the compartment. In this case, the patient should open the inhaler and, by lifting the capsule, remove it from the compartment. The patient should NOTlift the capsule by pressing the buttons multiple times. Then, the patient should repeat the steps described in point 7.
- 8. After hearing the characteristic sound, the patient should hold their breath for as long as possible
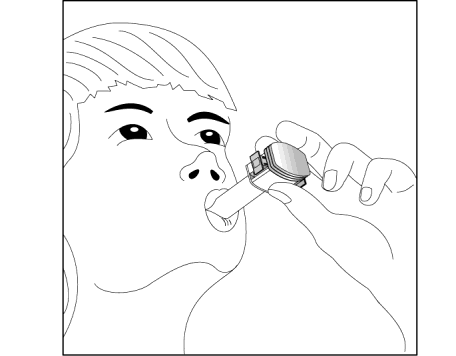
without feeling uncomfortable and then remove the inhaler from their mouth. The patient should exhale through their nose. Open the inhaler and check if there is still powder in the capsule. If there is powder left in the capsule, the patient should repeat the steps described in points 6 to 8.
- 9. After use, open the inhaler, remove the empty capsule, close the mouthpiece, and put the cap back on.
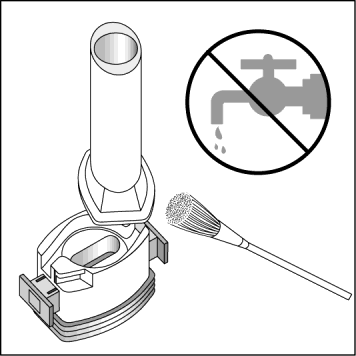
Cleaning the inhaler:
To remove powder residues, the patient should wipe the mouthpiece and capsule compartment with a DRYcloth. The patient can also use a clean, soft brush for this purpose. The patient should NOTuse water to clean the inhaler.
Using a higher dose of Foradil than recommended
If the patient has accidentally taken a higher dose of Foradil than recommended, they should immediately consult their doctor or pharmacist. The occurrence of nausea and/or vomiting, muscle tremors, headache, dizziness (possible symptoms of high blood pressure), rapid or irregular heartbeat, drowsiness, increased blood glucose levels, decreased blood potassium levels may indicate that the dose of Foradil is too high. The patient should immediately inform their doctor or go to the emergency room of the nearest hospital. The patient may require appropriate treatment.
Missing a dose of Foradil
If the patient misses a dose, they should take it as soon as possible. If it is almost time for the next scheduled dose, the patient should not take a double dose (to make up for the missed dose) but should return to their regular dosing schedule. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Foradil can cause side effects, although not everybody gets them. In clinical trials with Foradil, severe asthma attacks (shortness of breath, cough, wheezing, and chest tightness) have been observed, which may require hospital treatment.
Severe side effects
The patient should stop using the medicine immediately and inform their doctor if they experience any of the following side effects:
Uncommon(may occur in less than 1 in 100 patients):
- bronchial constriction with cough, wheezing, and difficulty breathing. Rare(may occur in less than 1 in 1000 patients):
- allergic reactions, e.g., rash, urticaria, itching, facial swelling, throat swelling, and low blood pressure, and bronchial constriction, Very rare(may occur in less than 1 in 10,000 patients):
- severe, described as tearing, chest pain (symptoms of angina pectoris), changes in the ECG, Frequency not known(frequency cannot be estimated from the available data):
- muscle weakness, muscle cramps, and heart rhythm disorders (these symptoms may be caused by low blood potassium levels),
- irregular heart rhythm (including faster heartbeat).
Other side effects
If any of the following side effects worsen, the patient should tell their doctor, pharmacist, or nurse.
Common side effects(may occur in less than 1 in 10 patients):
headache, muscle tremors, palpitations.
Uncommon side effects(may occur in less than 1 in 100 patients):
agitation, anxiety, nervousness, insomnia, dizziness, rapid heartbeat, throat irritation, dry mouth, muscle cramps, muscle pain.
Rare side effects(may occur in less than 1 in 1000 patients):
low blood potassium levels, heart rhythm disorders, extra heartbeats, nausea.
Very rare side effects(may occur in less than 1 in 10,000 patients):
high blood glucose levels, taste disorders, swelling of the hands, feet, and ankles, excessive thirst, frequent urination, fatigue (these symptoms may indicate high blood glucose levels).
Frequency not known(frequency cannot be estimated from the available data):
cough, rash, headache, and dizziness (possible symptoms of high blood pressure).
Using Foradil may cause an increase in blood insulin levels, free fatty acids, glycerol, and ketone bodies.
In some patients, Foradil may cause other side effects not listed in this leaflet. If the patient experiences any side effects not listed in this leaflet, they should tell their doctor or pharmacist.
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C 02-222 Warsaw tel. +48 22 49 21 301 fax: +48 22 49 21 309 website: https://smz.ezdrowie.gov.pl . Side effects can also be reported to the marketing authorization holder. By reporting side effects, it is possible to gather more information on the safety of this medicine.
5. How to store Foradil
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and blister pack after: EXP. The expiry date refers to the last day of the month. Store in a temperature below 25°C. Store in the original packaging to protect from moisture. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Foradil contains
- The active substance of Foradil is formoterol fumarate dihydrate. One hard capsule contains 12 micrograms of formoterol fumarate dihydrate.
- The other ingredients are: lactose monohydrate (25 mg per capsule, contains milk proteins), gelatin, and ink of the following composition: shellac, iron oxide black (E 172), propylene glycol, ammonia hydroxide 28%.
What Foradil looks like and contents of the pack
Foradil is a powder for inhalation in capsules. The powder in the capsule is intended for inhalation into the lungs using an inhaler called Aerolizer. Foradil is available in packs containing 30 or 60 capsules in blisters and an inhaler or 180 capsules and 3 inhalers (3 packs of 60 capsules in blisters and an inhaler in each pack)
Marketing authorization holder
Sandoz GmbH Biochemiestrasse 10 6250 Kundl Austria
Manufacturer/Importer
Novartis Pharma GmbH Roonstrasse 25 90429 Nürnberg Germany Novartis Farmacéutica S.A. Gran Via de les Corts Catalanes, 764 08013 Barcelona Spain Novartis Poland Sp. z o.o. ul. Marynarska 15 02-674 Warszawa Salutas Pharma GmbH Otto-von-Guericke Allee 1, 39179 Barleben, Germany Date of last revision of the leaflet:07/2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterNovartis Farmacéutica, S.A. Novartis Pharma GmbH Novartis Poland Sp. z o.o. Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ForadilDosage form: Aerosol, 12 mcg/measured doseActive substance: formoterolManufacturer: Chiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbHPrescription requiredDosage form: Powder, 12 mcg/inh. doseActive substance: formoterolPrescription requiredDosage form: Powder, 12 mcgActive substance: formoterolPrescription required
Alternatives to Foradil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Foradil in Ukraine
Alternative to Foradil in Spain
Online doctors for Foradil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Foradil – subject to medical assessment and local rules.














