
OXIS TURBUHALER 9 micrograms POWDER FOR INHALATION

How to use OXIS TURBUHALER 9 micrograms POWDER FOR INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Oxis Turbuhaler 9 micrograms/inhalation powder for inhalation
Formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
- What Oxis Turbuhaler is and what it is used for
- What you need to know before you use Oxis Turbuhaler
- How to use Oxis Turbuhaler
- Possible side effects
- Storing Oxis Turbuhaler
- Contents of the pack and other information
1. What Oxis Turbuhaler is and what it is used for
Oxis Turbuhaler is an inhaler and contains the active substance called formoterol, which belongs to a group of medicines called long-acting beta2-adrenergic agonists or bronchodilators.
Formoterol works by relaxing the muscles in the airways, making it easier to breathe. The effect starts within 1 to 3 minutes and lasts for up to 12 hours.
Your doctor has prescribed this medicine to treat asthma or chronic obstructive pulmonary disease (COPD).
Asthma:
Oxis Turbuhaler is used to treat asthma in adults, adolescents, and children over 6 years of age.
For asthma, your doctor will prescribe two inhalers: Oxis Turbuhaler and a separate steroid inhaler. Both should be used together.
- Oxis Turbuhaler is used to help prevent asthma symptoms from occurring.
- Some patients also use Oxis Turbuhaler when they need extra doses to relieve asthma symptoms and to help them recover their breathing.
- Oxis Turbuhaler can also be used before exercise to prevent asthma symptoms caused by exercise.
Chronic Obstructive Pulmonary Disease (COPD):
Oxis Turbuhaler can also be used to treat the symptoms of COPD in adults. COPD is a long-term disease of the airways in the lungs, often caused by smoking.
2. What you need to know before you use Oxis Turbuhaler
Do not use Oxis Turbuhaler:
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before starting to use Oxis Turbuhaler if:
- You have diabetes; you may need to have your blood glucose levels checked more often while using this medicine.
- You have high blood pressure or have ever had heart problems.
- You have thyroid problems.
- You have low potassium levels in your blood; your doctor may ask you to have blood tests to check your potassium levels.
- You have severe liver problems, such as liver cirrhosis.
If you are not sure if any of these situations apply to you, ask your doctor or pharmacist before using Oxis Turbuhaler.
Using Oxis Turbuhaler with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines that you buy without a prescription and herbal medicines, as Oxis Turbuhaler may affect the way that some other medicines work, and some medicines may affect the way that Oxis Turbuhaler works.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- Beta-blockers (such as atenolol or propranolol for high blood pressure), including eye drops (such as timolol for glaucoma).
- Medicines for an irregular heartbeat (such as quinidine).
- Medicines like digoxin, which is normally used to treat heart failure.
- Diuretics (such as furosemide), used to treat high blood pressure.
- Corticosteroids taken by mouth (such as prednisolone).
- Xanthines (such as theophylline or aminophylline), which are normally used to treat asthma.
- Erythromycin (used to treat infections).
- Antihistamines (such as terfenadine).
- Other substances that dilate the airways (bronchodilators, such as salbutamol).
- Ephedrine (used to treat asthma or as a decongestant).
- Tricyclic antidepressants (such as amitriptyline).
If any of these situations apply to you, or if you are not sure, ask your doctor or pharmacist before using Oxis Turbuhaler.
The addition of anticholinergics (such as tiotropium bromide or ipratropium bromide) to the treatment with Oxis Turbuhaler may help to open your airways even more.
Also, tell your doctor or pharmacist if you are going to have a general anesthetic for an operation or for dental treatment.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine; do not use it unless your doctor tells you to.
- If you become pregnant while using Oxis Turbuhaler, do not stop using it, but talk to your doctor immediately.
Driving and using machines
Oxis Turbuhaler is unlikely to affect your ability to drive or use tools or machines.
Oxis Turbuhaler contains lactose
Oxis Turbuhaler contains lactose, which is a type of sugar. If your doctor has told you that you have an intolerance to some sugars, contact them before using this medicine. The amount of lactose in this medicine is normally not enough to cause problems in people with lactose intolerance.
The excipient lactose contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Oxis Turbuhaler
- Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
- Do not use more doses than prescribed by your doctor without talking to them first.
- If you are using Oxis Turbuhaler regularly for asthma or COPD, you should continue to use it even if you do not have any symptoms.
Important information about asthma or COPD symptoms
If while using Oxis Turbuhaler you feel difficulty breathing or wheezing, you should continue to use it and see your doctor as soon as possible, as you may need additional treatment.
Contact your doctor immediately if:
- Your breathing gets worse, or if you wake up at night with asthma symptoms.
- You notice that you are feeling tightness in the chest.
- You do not feel relief with your usual dose.
- You need to use more doses than usual, for more than 2 days a week.
- You need to use the Turbuhaler more often than usual before exercise.
These signs may indicate that your asthma or COPD is not being adequately controlled and that you need a different or additional treatment immediately.
Asthma
Oxis Turbuhaler should not be used in children under 6 years of age.
Adults (18 years and older)
- The recommended dose is 1 inhalation, 1 or 2 times a day.
- Your doctor may increase the dose to 2 inhalations, 1 or 2 times a day.
- Some patients also use Oxis Turbuhaler for relief of symptoms. If you have asthma symptoms, the usual dose is 1 inhalation when symptoms occur.
- Normally, no more than a total of 4 inhalations per day are needed. This includes the inhalations you do daily, when you have asthma symptoms, and before exercise. However, your doctor may allow up to 6 inhalations per day, although no more than 6 inhalations should be taken in 24 hours.
- Do not take more than 3 inhalations at one time.
Children and adolescents (6 to 17 years)
- The recommended dose is 1 inhalation, 1 or 2 times a day.
- Some children also use Oxis Turbuhaler for relief of symptoms. If they have asthma symptoms, the usual dose is 1 inhalation when symptoms occur.
- Normally, no more than a total of 2 inhalations per day are needed. This includes the inhalations they do daily, when they have asthma symptoms, and before exercise. However, the doctor may allow up to 4 inhalations per day, although no more than 4 inhalations should be taken in 24 hours.
- Children should not take more than 1 inhalation at one time.
Your doctor (or nurse) will help you to manage your asthma. Once good control of your asthma has been achieved, your doctor may consider it appropriate to gradually reduce the dose of Oxis Turbuhaler.
Exercise-induced asthma
Both in children and adults, if you have asthma symptoms caused by exercise, your doctor may tell you to also use Oxis Turbuhaler before exercise. Oxis Turbuhaler should not be used in children under 6 years of age.
Adults (18 years and older)
- The recommended dose is 1 inhalation before exercise.
- Normally, no more than a total of 4 inhalations per day are needed. This includes the inhalations you do daily, when you have asthma symptoms, and before exercise. However, your doctor may allow up to 6 inhalations per day, although no more than 6 inhalations should be taken in 24 hours.
- Do not take more than 3 inhalations at one time.
Children and adolescents (6 to 17 years)
- The recommended dose is 1 inhalation before exercise.
- Normally, no more than a total of 2 inhalations per day are needed. This includes the inhalations they do daily, when they have asthma symptoms, and before exercise. However, the doctor may allow up to 4 inhalations per day, although no more than 4 inhalations should be taken in 24 hours.
- They should not take more than 1 inhalation at one time.
Chronic Obstructive Pulmonary Disease (COPD)
- It should only be used in adults (18 years and older).
- The recommended dose is 1 inhalation, 1 or 2 times a day.
- Your doctor may tell you to use extra doses to relieve your COPD symptoms.
- Do not use more than 4 inhalations per day.
- Do not take more than 2 inhalations at one time.
How to prepare your new Oxis Turbuhaler inhaler
Before using your newOxis Turbuhaler inhaler for the first time, you must prepare the inhaler for use as follows:
- Twist and lift the cover. You may hear a rattling sound.
- Hold the inhaler upright with the turquoise base at the bottom.
- Turn the turquoise base as far as it will go in one direction. Then turn it as far as it will go in the other direction (it does not matter which direction you turn it first).
- You should hear a click.
- Repeat this process once more, turning the turquoise base in both directions.
- Your Oxis Turbuhaler inhaler is now ready to use.
How to take an inhalation
Each time you need to take an inhalation, follow the instructions below:
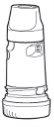
1. Twist and lift the white cover. You may hear a rattling sound.
2. Hold the inhaler uprightwith the turquoise base at the bottom.
3. Do not hold the mouthpiece while loading the Turbuhaler. To load the Turbuhaler with a dose, turn the turquoise base as far as it will go in one direction and then turn it as far as it will go in the other direction (it does not matter which direction you turn it first). You should hear a click. At this point, the Turbuhaler is loaded and ready to use. Load the Turbuhaler only when you need to use it.
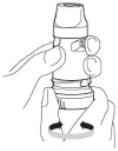
4. Hold the inhaler away from your mouth. Breathe out gently (as much as you can without feeling uncomfortable). Do not breathe out through the Turbuhaler.
5. Place the mouthpiece gently between your teeth, close your lips, and breathe in as forcefully and deeply as you can. Do not bite or press the mouthpiece with your teeth.
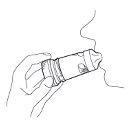
6. Remove the inhaler from your mouth and then breathe out gently. The amount of medicine released is very small, so you may not taste it after inhalation. However, if you have followed the instructions for use, you can be sure that you have inhaled the dose and that the medicine has reached your lungs.
7. If you need to take a second inhalation, repeat steps 2 to 6.
8. Replace the cover firmly after use.
Do not try to separate the mouthpiece as it is fixed to the Turbuhaler and should not be separated. The mouthpiece can be rotated but should not be turned unnecessarily. Do not use the Turbuhaler if it is damaged or if the mouthpiece has come off the Turbuhaler.
As with other inhalers, caregivers should ensure that children who have been prescribed Oxis Turbuhaler use the inhalation technique correctly, as described above.
Cleaning the Turbuhaler
Clean the outside of the mouthpiece once a week with a dry cloth; do not use water or liquids.
When to start a new Turbuhaler
- The dose indicator shows how many doses are left in the Turbuhaler, starting from 60 doses when it is full.
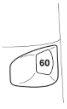
- The dose indicator is marked in intervals of 10 doses, so not all doses are shown. When you first see a red mark at the end of the dose indicator window, there are approximately 20 doses left. During the last 10 doses, the background of the dose indicator is red. When the "0" at the bottom of the red dose indicator window reaches the center of the window, you should start a new Turbuhaler.
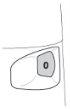
Note:
- The base may continue to turn and click even when the Turbuhaler is empty.
- The sound you hear when shaking the inhaler is made by a desiccant and not by the medicine. Therefore, the sound does not indicate how much medicine is left in the Turbuhaler.
- If you load your Oxis Turbuhaler inhaler more than once by mistake before taking your dose, you will only inhale one dose. However, the dose indicator will register all the doses loaded.
If you use more Oxis Turbuhaler than you should
If you use more Oxis Turbuhaler than you should, talk to your doctor or pharmacist immediately. You may experience the following effects: trembling, headache, or rapid heartbeat.
If you forget to use Oxis Turbuhaler
- If you forget to take a dose, take it as soon as you remember, although if it is almost time for your next dose, skip the missed dose.
- Do nottake a double dose to make up for a missed dose.
If you stop using Oxis Turbuhaler
Do not stop using Oxis Turbuhaler without talking to your doctor.
If you have any further questions on how to use your Turbuhaler, ask your doctor, nurse, or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you experience the following, stop using Oxis Turbuhaler and contact your doctor immediately:
- Bronchospasm (constriction of the muscle of the airways that causes sudden wheezing with breathing) after inhalation. This occurs very rarely, and may affect up to 1 in 10,000 people.
Other Possible Adverse Effects
Frequent (may affect up to 1 in 10 people)
-Tremors. If these effects appear, they are generally mild and disappear while continuing treatment with Oxis Turbuhaler.
- Headache.
- Dizziness.
- Nausea (feeling of discomfort).
- Muscle cramps.
Infrequent(may affect up to 1 in 100 people)
- Palpitations (noticing heartbeats). If this effect appears, it is generally mild and disappears while continuing treatment with Oxis Turbuhaler.
- Difficulty sleeping.
- Rapid heartbeats.
- Allergic reactions such as rash, itching, or bronchospasm.
- Low potassium levels in the blood.
- High blood sugar (glucose) levels.
- Alterations in taste, such as unpleasant mouth taste.
- Alterations in blood pressure.
- Irregular heartbeats.
- Chest pain or tightness (angina pectoris).
Rare (may affect up to 1 in 1,000 people)
- Feeling of agitation and restlessness.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Oxis Turbuhaler
- Keep this medicine out of sight and reach of children.
- This medicine does not require special storage conditions.
- When not in use, Oxis Turbuhaler should be kept with the cap firmly closed.
- Do not use this medicine after the expiration date shown on the packaging or on one side of the Turbuhaler label. The expiration date refers to the last day of that month.
- You must always ensure that you dispose of your used Turbuhaler responsibly, as part of the medicine will remain inside. Medicines should not be thrown away through drains or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Oxis Turbuhaler
The active ingredient is formoterol fumarate dihydrate. Each dose contains 12 micrograms of formoterol fumarate dihydrate, of which 9 micrograms can be inhaled.
The excipient is lactose monohydrate (which contains milk proteins). See section 2, Oxis Turbuhaler contains lactose.
Appearance of the Product and Package Contents
Oxis Turbuhaler is an inhaler that contains your medicine. The inhalation powder is white. Each Turbuhaler contains 60 doses, with the inhaler body being white and the turbine being turquoise.
Oxis Turbuhaler is available in the following package sizes: 60 doses (1 inhaler), 3x60 doses (3 inhalers), 10x60 doses (10 inhalers), 18x60 doses (18 inhalers), or 20x60 doses (20 inhalers). Not all sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Laboratory Beta, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
Manufacturer:
AstraZeneca AB
Forskargatan 18
SE-151 36 Södertälje
Sweden
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany Oxis Turbohaler 12 Mikrogramm Pulver zur Inhalation
Austria Oxis Turbohaler 12 μg – Dosier - Pulverinhalator
Belgium Oxis Turbohaler 9 μg/dose
Denmark Oxis Turbuhaler
Spain Oxis Turbuhaler 9 micrograms/inhalation powder for inhalation
Finland Oxis Turbuhaler
Greece Oxez Turbuhaler
Netherlands Oxis 12 Turbuhaler
Ireland Oxis Turbohaler 12 inhalation powder
Italy Oxis Turbohaler 9
Luxembourg Oxis Turbohaler 9 μg/dose
Portugal Oxis Turbohaler
Sweden Oxis Turbuhaler
UK Oxis Turbohaler 12 inhalation powder
Date of the Last Revision of this Prospectus:March 2020
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price22.68 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OXIS TURBUHALER 9 micrograms POWDER FOR INHALATIONDosage form: PULMONARY INHALATION, 12 mcg/actuationActive substance: formoterolManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, 12 mcg formoterol fumarateActive substance: formoterolManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 12 microgramsActive substance: formoterolManufacturer: Viatris Healthcare LimitedPrescription required
Online doctors for OXIS TURBUHALER 9 micrograms POWDER FOR INHALATION
Discuss questions about OXIS TURBUHALER 9 micrograms POWDER FOR INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















