
FORMOTEROL STADA 12 micrograms powder for inhalation (hard capsule)

How to use FORMOTEROL STADA 12 micrograms powder for inhalation (hard capsule)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Formoterol STADA 12 micrograms inhalation powder (hard capsule)
formoterol fumarate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Formoterol Stada and what is it used for
- What you need to know before you use Formoterol Stada
- How to use Formoterol Stada
- Possible side effects
- Storing Formoterol Stada
- Contents of the pack and further information
1. What is Formoterol Stada and what is it used for
Formoterol Stada contains a substance called formoterol fumarate dihydrate. It belongs to a group of medicines called “bronchodilators” or “long-acting beta2 agonists”.
This medicine is used:
- Together with inhaled corticosteroids as maintenance treatment for moderate to severe asthma.
- To prevent breathing difficulties caused by inhaling allergens (substances that cause allergy), cold air, or exercise.
- As maintenance treatment to relieve symptoms in patients with chronic obstructive pulmonary disease (COPD).
2. What you need to know before you use Formoterol Stada
Follow your doctor's or pharmacist's instructions carefully, even if they are different from those contained in this leaflet.
Do not use Formoterol Stada:
- If you are allergic to formoterol or any of the other ingredients of this medicine (listed in section 6).
Talk to your doctor about the risks and benefits of treating your asthma with this medicine.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Formoterol Stada.
Be particularly careful with this medicine:
- If you have any heart disease or any abnormality of the type “prolongation of the QT interval” (abnormal electrical signal detected in your electrocardiogram).
- If you have high blood pressure.
- If you have hyperthyroidism (disease of the thyroid gland).
- If you have an aneurysm (localized dilation of an artery caused by degeneration or weakening of the vascular wall).
- If you are diabetic or if your doctor has told you that you have intolerance to some sugars, consult your doctor before taking this medicine.
Treatment with formoterol may lead to an increase in blood sugar levels. Therefore, if you are diabetic, you will need to monitor your blood sugar levels.
- If you have a pheochromocytoma (a tumor of the adrenal gland that can affect blood pressure).
- If you have asthma, do not use Formoterol Stada as your only medicine for asthma. Use this medicine only with an inhaled corticosteroid (ICS).
- If you feel that you have difficulty breathing or wheezing (whistling) during the use of this medicine, you should continue using Formoterol Stada, but you should see your doctor as soon as possible, as you may need additional treatment.
Do not use this medicine to relieve sudden wheezing (whistling). Always carry a rescue inhaler, such as salbutamol (short-acting beta2 agonist), to treat sudden asthma symptoms.
Important information about this medicine
- Do not change or stop any of your treatments for controlling or treating your respiratory problems, including inhaled corticosteroids. Your doctor will adjust the treatment according to your needs.
- Once your asthma is well controlled, your doctor may consider it appropriate to gradually reduce the dose of this medicine.
- If you are well controlled with an inhaled corticosteroid, you should not use this medicine.
- Do not use this medicine if you only need, from time to time, a medicine like salbutamol (short-acting beta2 agonist).
- While using Formoterol Stada, do not use other medicines with a similar effect, such as salmeterol (long-acting beta2 agonists).
- Do not start treatment with this medicine or increase the recommended dose by your doctor while you are having an asthma attack.
Treatment with formoterol may produce a low level of potassium in the blood. This can make you more susceptible to an abnormal heart rhythm. Therefore, your doctor may need to check your potassium level in the blood, especially if you have severe asthma.
Consult your doctor if you have any doubts about how Formoterol Stada works or why it has been prescribed for you.
Patients over 65 years of age
If you are 65 years of age or older, you can use this medicine at the same dose as adults.
Children and adolescents
Formoterol Stada is not suitable for children under 6 years of age.
Children from 6 years of age should only use this medicine if they are able to handle it correctly (see section "How to use the capsules of this medicine with your inhaler"). They should only use the inhaler under adult supervision.
Other medicines and Formoterol Stada
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This is especially important if you are taking any of the following medicines:
- Monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants, which are medicines used to treat depression and mood disorders.
- Sympathomimetic agents, which are medicines similar to adrenaline and are used to treat asthma and nasal congestion.
- Antihistamines, which are commonly used to prevent or treat the main symptoms of an allergic reaction.
- Steroids, which are often used to treat asthma and other anti-inflammatory diseases.
- Diuretics, which are used to treat edema (water retention), heart failure, and high blood pressure.
- Beta-blockers, which are used to treat high blood pressure, heart failure, angina, anxiety, and abnormal heart rhythm. Certain eye drops used to treat glaucoma may contain beta-blockers.
- Quinidine, disopyramide, and procainamide, which are used to treat abnormal heart rhythm.
- Phenothiazine derivatives, which are used to control mental disorders such as schizophrenia, mania, psychotic states, and anxiety.
- Digitalis, which is used to treat heart failure and abnormal heart rhythm.
- Xanthine derivatives, which are used to treat asthma and chronic obstructive pulmonary diseases.
- Macrolides (e.g., erythromycin) used to treat bacterial infections.
- Anesthetics such as halogenated hydrocarbons (e.g., halothane) used to produce anesthesia during surgery.
- Anticholinergics (e.g., ipratropium bromide) used to treat gastrointestinal, genitourinary, and other disorders.
It may be necessary for your doctor to change or even stop the dose of one of the medicines.
If your doctor has prescribed you other medicines that you must take regularly to treat your respiratory disease, it is essential that you continue to take them as usual and do NOT STOP or reduce the dose, even if you start to feel much better.
Pregnancy, breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Do not use this medicine during pregnancy unless your doctor tells you to. He or she will inform you of the potential risk of using formoterol during pregnancy.
Breastfeeding
Mothers who are taking this medicine should not breastfeed.
Driving and using machines
In some patients, this medicine may cause dizziness. If you feel dizzy, do not drive, use machines, or perform any activity that requires your attention.
Formoterol Stada contains lactose
It may cause allergic reactions in patients with cow's milk protein allergy.
If your doctor has told you that you have an intolerance to certain sugars, consult with him before taking this medicine.
Use in athletes
Athletes are informed that this medicine contains a component that may result in a positive doping test.
3. How to use Formoterol Stada
Follow the instructions for administration of this medicine contained in this leaflet or as indicated by your doctor. In case of doubt, consult your doctor or pharmacist.
Your doctor will tell you the amount and frequency of use of Formoterol Stada according to your needs. Do not exceed the recommended dose. Remember to use your medicine.
The effect of the dose lasts up to 12 hours.
Do not swallow the capsules. They must be used by inhaling the contents with the inhalation device.
Use in adults (including elderly)
Maintenance treatment of asthma
For asthma treatment, you will always be prescribed Formoterol Stada together with an inhaled corticosteroid.
- The recommended dose is 1 capsule inhaled twice a day. In more severe cases, 2 capsules may be administered twice a day.
- In addition to the normal dose, you may occasionally need 1 or 2 additional capsules per day to relieve usual symptoms. If you need to take these additional doses more than twice a week, consult your doctor, as it may indicate a worsening of the disease.
- The maximum recommended daily dose for adults is 4 capsules.
Prevention of breathing difficulties caused by inhaling allergens, cold air, or exercise
- The recommended dose is 1 capsule inhaled at least 15 minutes before exercise or exposure to the allergen or cold air.
- In some cases, your doctor may advise you to use 2 capsules to prevent wheezing and bronchospasm.
Maintenance treatment of COPD (chronic obstructive pulmonary disease)
- The recommended dose is 1 capsule inhaled twice a day, which must be administered with the inhaler as described in the section “How to use the capsules of this medicine with your inhaler”. In more severe cases, 2 capsules may be administered twice a day.
Use in children and adolescents (from 6 years of age)
This medicine is not recommended for children under 6 years of age. See section “Children and adolescents” in section 2. What you need to know before you use Formoterol Stada.
Maintenance treatment of asthma
- The recommended dose is 1 capsule inhaled twice a day.
- The maximum recommended daily dose is 2 capsules per day.
Prevention of breathing difficulties caused by inhaling allergens, cold air, or exercise
- The recommended dose is 1 capsule inhaled at least 15 minutes before exercise or exposure to the allergen or cold air.
How to use the capsules of this medicine with your inhaler
Follow the instructions below to learn how to use this medicine.
Use the capsules of this medicine only with the inhalerprovided in the package. The inhaler included in the package has been specially developed for use with this medicine.
Remove the capsule from the blister just before use. Make sure your fingers are completely dry so that the capsule does not become damp.
Do not swallow the capsule. The powder in the capsule is for inhalation only.
Instructions for correct use
1. Remove the protective cap.
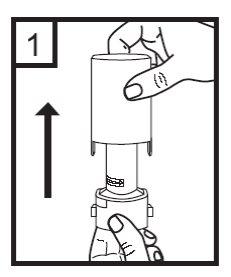
2. Hold the base of the inhaler firmly and turn the mouthpiece in the direction of the arrow to open the device.
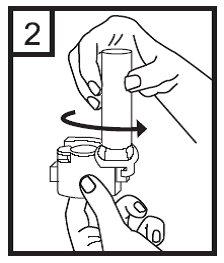
3. Place the capsule in the compartment located at the base of the inhaler. It is essential not to remove the capsule from the package until the moment of use.
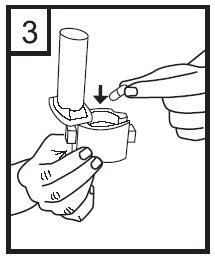
4. Turn the mouthpiece to the closed position.
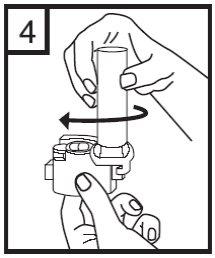
5. Press the red buttons while keeping the inhaler in a vertical position. Release the buttons.
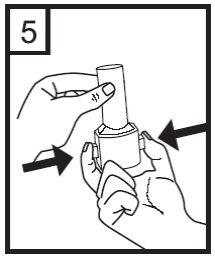
6. Exhale completely.
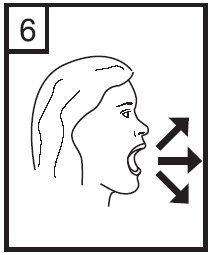
7. Insert the mouthpiece into your mouth and tilt your head slightly backward. Press the mouthpiece with your lips and breathe in quickly and constantly with the greatest depth possible.
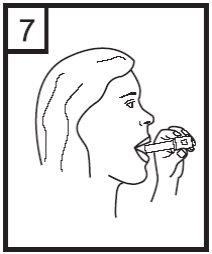
8. Hold your breath for as long as possible without feeling uncomfortable while removing the inhaler from your mouth. Then exhale. Open the inhaler to see if there is powder left in the capsule. If so, repeat steps 6 to 8.
9. After use, remove the empty capsule, close the mouthpiece, and replace the cap.
Cleaning the inhaler
To remove residual powder, clean the mouthpiece and capsule compartment with a dry cloth.
A soft, clean brush can also be used.
If you use more Formoterol Stada than you should
If you accidentally use more formoterol than your doctor prescribed, you may feel nausea or vomiting, or you may experience tremors, headache, dizziness (possible symptoms of high blood pressure), rapid heartbeat, or drowsiness. Inform your doctor immediately or go to the nearest emergency department. You may need medical attention.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone 91 562 04 20, indicating the medicine and the amount taken. It is recommended to take the package and leaflet of the medicine to the healthcare professional.
If you forget to use Formoterol Stada
Do not take a double dose to make up for forgotten doses. If you forget a dose, use it as soon as you remember. If it is almost time for the next dose, skip that dose and continue with your usual dosing schedule.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
In some situations, severe asthma attacks have been observed (severe increase in shortness of breath, cough, wheezing, or chest tightness, which can lead to hospitalization).
Some effects can be serious:If you experience any of these adverse effects, inform your doctor immediately:
Rare adverse effects: may affect up to 1 in 1,000 people.
- Bronchospasm (contraction of the bronchi causing difficulty breathing) with wheezing or cough and difficulty breathing.
- Symptoms including muscle weakness, muscle spasms, and/or abnormal heart rhythm (this could mean you have low potassium levels in your blood).
- Irregular heartbeat (including rapid heartbeat).
Very rare adverse effects: may affect up to 1 in 10,000 people.
- Chest pain of an oppressive nature (symptom of angina pectoris).
- Allergic reactions, for example, if you feel weak (have low blood pressure), if you develop a skin rash or experience itching or swelling of the face.
Other Adverse Effects:
Frequent adverse effects: may affect up to 1 in 10 people.
- Headache
- Tremor
- Palpitations
Infrequent adverse effects: may affect up to 1 in 100 people.
- Agitation
- Anxiety
- Nervousness
- Sleep disorders
- Rapid heartbeats
- Throat irritation
- Dry mouth
- Muscle cramps
- Muscle pain
- Dizziness
Rare adverse effects: may affect up to 1 in 1,000 people.
- Nausea
Very rare adverse effects: may affect up to 1 in 10,000 people.
- Alteration of taste
- Swelling of the hands, ankles, or feet
- Excessive thirst, frequent urination, and fatigue over a long period (a possible indication of high blood sugar levels)
- Prolongation of the QT interval
Frequency not known: cannot be estimated from the available data.
- Cough
- Skin rash
- Headache and dizziness (symptoms of high blood pressure)
Some of these adverse effects may disappear spontaneously
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Formoterol Stada
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging, after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
Keep in the original packaging to protect it from moisture.
Always discard the old inhaler and use the new one provided in the packaging.
Medicines should not be thrown away through the sewers or in the trash. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Formoterol Stada
- The active ingredient is formoterol fumarate dihydrate. Each hard capsule contains 12 micrograms of formoterol fumarate (as formoterol fumarate dihydrate).
- The other components are micronized lactose monohydrate, semi-micronized lactose monohydrate, and hard gelatin capsules.
Appearance of the Product and Package Contents
Formoterol Stada 12 micrograms inhalation powder (hard capsule), are colorless and transparent capsules that contain white powder and must be used with the inhaler provided in the packaging.
Each package contains 60 hard capsules.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratory STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Spain
Manufacturer
Liconsa, S.A.
Avda. Miralcampo, Nº 7, Pol. Ind. Miralcampo
19200 Azuqueca de Henares (Guadalajara)
Spain
Date of the Last Revision of this Leaflet:May 2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Inhaler CE. The inhalation device complies with Directive 93/42/CEE.
- Country of registration
- Average pharmacy price22.54 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FORMOTEROL STADA 12 micrograms powder for inhalation (hard capsule)Dosage form: PULMONARY INHALATION, 12 mcg/actuationActive substance: formoterolManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, 12 mcg formoterol fumarateActive substance: formoterolManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 12 microgramsActive substance: formoterolManufacturer: Viatris Healthcare LimitedPrescription required
Online doctors for FORMOTEROL STADA 12 micrograms powder for inhalation (hard capsule)
Discuss questions about FORMOTEROL STADA 12 micrograms powder for inhalation (hard capsule), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















