
Emla

Ask a doctor about a prescription for Emla

How to use Emla
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
EMLA, 25 mg/g + 25 mg/g, cream
Lidocaine + Prilocaine
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is EMLA and what is it used for
- 2. Important information before using EMLA
- 3. How to use EMLA
- 4. Possible side effects
- 5. How to store EMLA
- 6. Contents of the packaging and other information
1. What is EMLA and what is it used for
EMLA contains two active substances - lidocaine and prilocaine. They belong to a group of medicines called local anesthetics.
The action of EMLA is to temporarily numb the sensation in the superficial layers of the skin. The cream is applied to the skin before certain medical procedures and treatments.
This helps to relieve pain in the skin; however, the patient may still feel pressure and touch.
Adults, adolescents, and children
EMLA can be used to numb the skin before:
- injecting a needle into the skin (e.g., when giving an injection or taking a blood sample)
- minor surgical procedures on the skin.
Adults and adolescents
EMLA can also be used:
- to numb the genital area before:
- injections,
- medical procedures such as removing warts. The application of EMLA cream to the genital area should be performed under the supervision of a doctor or nurse.
Adults
EMLA can also be used to numb the skin before:
- debridement or removal of damaged skin on lower limbs with ulcers.
2. Important information before using EMLA
When not to use EMLA:
- if the patient has been diagnosed with an allergy to lidocaine, prilocaine, another similar local anesthetic, or any of the other ingredients of this medicine (listed in section 6).
- 6.
Warnings and precautions
Before starting to use EMLA, you should discuss it with your doctor or pharmacist:
- if the patient has a rare metabolic disorder that affects the blood and is called glucose-6-phosphate dehydrogenase deficiency,
- if the patient has a problem with the concentration of a blood pigment called methemoglobinemia,
- you should not apply EMLA to areas of skin with a rash, cuts, scratches, or other open wounds, except for ulcers on the lower limbs. If the patient has any of these changes, they should contact their doctor or pharmacist before using the cream,
- if the patient has a skin disorder with itching called atopic dermatitis, it may be sufficient to use the cream for a shorter time. The use of the cream for more than 30 minutes is associated with a higher likelihood of a local skin reaction (see also section 4. "Possible side effects"),
- if the patient is taking certain medications used to treat heart rhythm disorders (anti-arrhythmic drugs of class III, such as amiodarone). In this case, the doctor will monitor the patient's heart activity.
Due to the possibility of increased absorption from freshly shaved skin, it is essential to follow the recommended dosage, area of application, and time of application on the skin.
You should avoid contact between EMLA and the eyes, as it may cause irritation and chemical burns to the eyes. If EMLA cream accidentally gets into the eye, it should be rinsed immediately with lukewarm water or a saline solution (0.9% NaCl solution).
You should be careful not to get anything into the eye until sensation returns.
You should closely monitor children when applying EMLA to any part of the body to prevent them from transferring EMLA to the eye (eyes).
You should not apply EMLA to a diseased eardrum.
When EMLA is used in a patient before administering a live vaccine (e.g., tuberculosis vaccine), they should remember to report for a follow-up visit at the time specified by the doctor to assess the effectiveness of the vaccination.
Children and adolescents
In infants and newborns under 3 months of age, a transient, clinically insignificant increase in methemoglobin concentration in the blood (a form of hemoglobin, or blood pigment) is commonly observed within 12 hours of applying EMLA.
The efficacy of EMLA during blood sampling from the heel in newborns or to ensure adequate pain relief during circumcision has not been confirmed in clinical trials.
EMLA should not be used on the mucous membranes of the genital area (e.g., vagina) in children (under 12 years of age) due to insufficient data on the absorption of active substances.
EMLA should not be used in children under 12 months of age who are being treated with other medications that affect blood pigment and may cause methemoglobinemia (e.g., sulfonamides; see also section 2 "EMLA and other medicines").
EMLA should not be used in premature newborns.
EMLA and other medicines
You should inform your doctor or pharmacist about any other medicines you are currently taking or have recently taken or may take. This includes medicines that can be bought without a prescription and herbal medicines. This is important because the ingredients of EMLA may affect the action of some other medicines, and some other medicines may affect the action of EMLA.
In particular, the patient should inform their doctor or pharmacist if they have used or taken any of the following medicines:
- Medicines used to treat infections called sulfonamides and nitrofurantoin.
- Medicines used to treat epilepsy: phenytoin and phenobarbital.
- Other local anesthetics.
- Medicines used to treat irregular heart rhythm, such as amiodarone.
- Cimetidine or beta-adrenergic blockers, which may increase the concentration of lidocaine in the blood. This interaction is not clinically significant in short-term use of EMLA in recommended doses.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Occasional use of EMLA during pregnancy is not associated with any risk of adverse effects on the fetus.
The active substances of EMLA (lidocaine and prilocaine) are excreted into breast milk. However, the amount that passes into milk is so small that there is essentially no risk to the breastfed child.
In animal studies, no fertility disorders were found in males or females treated with the active ingredients of EMLA.
Driving and using machines
EMLA has no influence or negligible influence on the ability to drive and use machines when used in recommended doses.
EMLA contains macrogolglycerol hydroxystearate
Macrogolglycerol hydroxystearate may cause skin reactions.
3. How to use EMLA
EMLA should always be used in accordance with the recommendations of a doctor, pharmacist, or nurse. If you have any doubts, you should consult a doctor, pharmacist, or nurse.
Using EMLA
- The site of application, amount of cream, and time of application depend on the purpose for which it is used.
- The doctor, pharmacist, or nurse will apply the cream to the appropriate area or show the patient how to do it themselves.
- When EMLA is used on the genital area, the doctor or nurse should supervise its use.
EMLA should not be used in the following areas:
- Areas of cuts, scratches, or open wounds, except for ulcers on the lower limbs.
- Areas with skin rash or eczema.
- Eyes or near the eyes.
- Inside the nose, ears, or mouth.
- Rectum.
- Genital area in children.
People who frequently apply or remove the cream from the patient's body should ensure that they effectively avoid contact with the cream to prevent the development of hypersensitivity.
The protective membrane of the tube is pierced using the tube cap.
Using on the skin before minor procedures (such as needle insertion or minor surgical procedures on the skin):
- The cream is applied to the skin in a thick layer. The doctor, pharmacist, or nurse will tell the patient where to apply the cream.
- Then, the layer of cream is covered with a dressing (plastic foil). The dressing is removed immediately before the procedure starts. If the patient applies the cream themselves, they should ensure that they have received dressings from their doctor, pharmacist, or nurse.
- Usually, the dose used in adults and adolescents over 12 years old is 2 g (grams).
- In adults and adolescents over 12 years old, the cream should be applied at least 60 minutes before the planned time of the procedure (except when the cream is to be applied to the genital area). However, the cream should not be applied more than 5 hours before the procedure or earlier.
- In children, the amount of EMLA cream used and the time of application depend on the child's age. The doctor, nurse, or pharmacist will inform the patient about the amount of cream to be used and when to apply it.
When applying the EMLA cream, it is very important to follow the instructions below carefully:
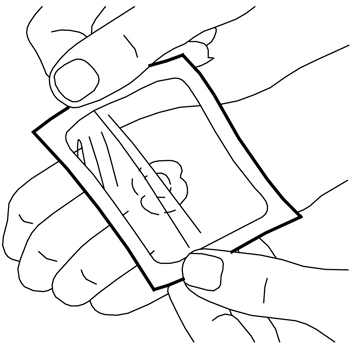
- 1. Squeeze out a portion of cream from the tube to form a mound in the area where it is needed on the skin (e.g., where the needle is to be inserted). A line of cream about 3.5 cm long from a 30 g tube corresponds to 1 g of cream. Do not rub the cream into the skin.
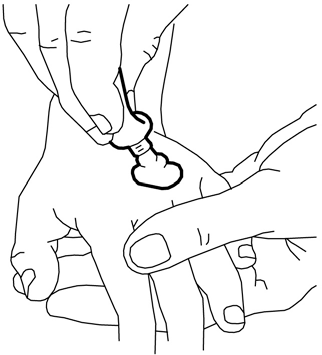
- 2. Peel off the paper layer from the middle of the non-sticky side of the dressing (leaving the paper frame).
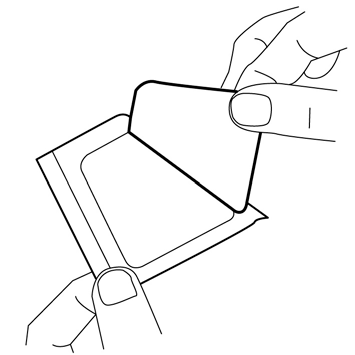
- 3. Remove the top layer of the adhesive dressing.
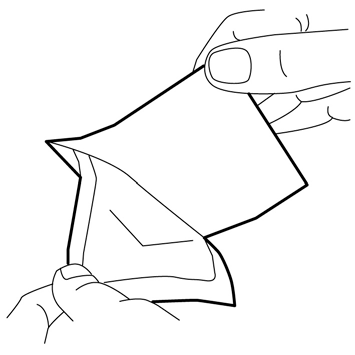
- 4. Carefully place the dressing over the mound of cream. Do not spread the cream under the dressing.
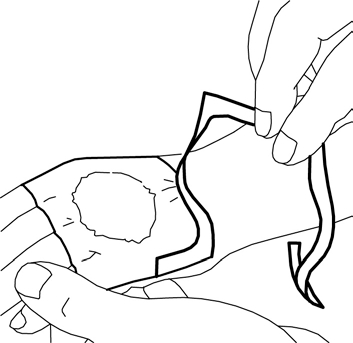
- 5. Remove the paper stiffener. Carefully smooth the edges of the dressing. Then, leave the dressing on for at least 60 minutes if the skin is not damaged. The cream should not be left on for more than 60 minutes in children under 3 months old or more than 30 minutes in children with atopic dermatitis. In the case of application to the genital area or ulcers, shorter application times may be used as described below.
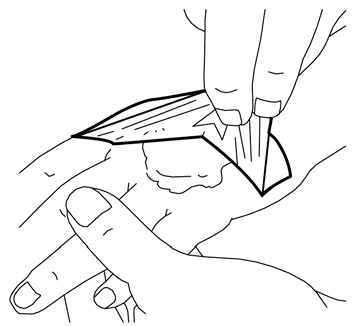
- 6. The doctor or nurse will remove the dressing and remove the cream immediately before the medical procedure (e.g., before inserting the needle).
Using on larger areas of freshly shaved skin before procedures in outpatient settings (such as hair removal):
Usually, the dose of EMLA used is 1 g of cream per 10 cm² (10 square centimeters) of skin surface, applied for 1 to 5 hours under a dressing. EMLA should not be used on an area of freshly shaved skin larger than 600 cm² (600 square centimeters, e.g., 30 cm x 20 cm). The maximum dose is 60 g.
Using on the skin before procedures in hospital settings (e.g., before skin grafting), which require deeper skin anesthesia:
- EMLA may be used in this way in adults and adolescents over 12 years old.
- Usually, the dose used is 1.5 g to 2 g of cream per 10 cm² (10 square centimeters) of skin surface.
- The cream is applied and covered with a dressing for 2 to 5 hours.
Using on the skin before removing warts
- EMLA may be used in children and adolescents with atopic dermatitis.
- Usually, the dose used depends on the child's age and is applied for 30 to 60 minutes (30 minutes in patients with atopic dermatitis). The doctor, nurse, or pharmacist will inform the patient about the amount of cream to be used.
Using on the genital skin before injecting local anesthetics
- EMLA may be used in this way only in adults and adolescents over 12 years old.
- Usually, the dose used is 1 g of cream (1 g to 2 g in the case of genital skin in women) per 10 cm² (10 square centimeters) of skin surface.
- The cream is applied and covered with a dressing. The dressing is left on for 15 minutes in men and 60 minutes in women.
on the genital skin.
Using on the genital skin before minor surgical procedures on the skin (such as removing warts)
- EMLA may be used in this way only in adults and adolescents over 12 years old.
- Usually, the dose used is 5 g to 10 g of cream for 10 minutes. No dressing is used. The procedure should be started immediately after.
Using on ulcers on the lower limbs before debridement or removal of damaged skin
- Usually, the dose used is 1 g to 2 g of cream per 10 cm² (10 square centimeters) of skin surface and no more than 10 g.
- The cream is applied and covered with a tight dressing, e.g., plastic foil. The cream and dressing are applied 30 to 60 minutes before the debridement procedure. The cream should be removed using a cotton swab and the debridement procedure started immediately.
- EMLA can be used before debridement of ulcers on the lower limbs up to 15 times over a period of 1-2 months.
- In the case of using the cream on ulcers on the lower limbs, the EMLA tube should be used as a single-use product: after each use of the cream in a patient, the tube with the remaining amount of cream should be discarded.
Using more EMLA than recommended
If more EMLA than recommended by the doctor, pharmacist, or nurse is used, they should be contacted immediately, even if the patient does not feel any discomfort.
Problems and discomfort that may occur after using too much EMLA are listed below. These discomforts should not occur when using EMLA according to the recommendations.
- Feeling of "emptiness" in the head or dizziness.
- Numbness or tingling of the skin around the mouth and tongue.
- Disturbed sense of taste.
- Blurred vision.
- Ringing in the ears.
- There is also a risk of methemoglobinemia (a problem with the concentration of blood pigment). This is more likely if the patient is taking certain other medicines. In the event of this condition, the skin becomes blue-gray due to insufficient oxygen in the blood.
In severe cases of overdose, symptoms such as seizures, decreased blood pressure, decreased breathing rate, cessation of breathing, and abnormal heart rhythm may occur. These problems can be life-threatening.
If you have any further doubts about using this medicine, you should consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, EMLA can cause side effects, although not everybody gets them.
If the patient experiences any of the following side effects, or any other side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- Local skin reactions (pale or red skin, slight swelling) are common and usually disappear without the need for medical treatment.
Frequent(may affect up to 1 in 10 people)
- Transient local skin reactions (pale or red skin, swelling) at the site of application during use on the skin, mucous membranes of the genital area, or ulcers on the lower limbs.
- Mild burning sensation, itching, or warmth at the site of application during use on the mucous membranes of the genital area or ulcers on the lower limbs.
Uncommon(may affect up to 1 in 100 people)
- Mild burning sensation, itching, or warmth at the site of application during use on the skin.
- Numbness (tingling) at the site of application during use on the mucous membranes of the genital area.
- Skin irritation at the site of application during use on ulcers on the lower limbs.
Rare(may affect up to 1 in 1,000 people)
- Allergic reactions, which in rare cases can lead to anaphylactic shock (skin rash, swelling, fever, difficulty breathing, and fainting) during use on the skin, mucous membranes of the genital area, or ulcers on the lower limbs.
- Methemoglobinemia (a blood disorder) during use on the skin.
- Minor pinpoint bleeding (petechiae) at the site of application (especially in children with eczema after longer application time) during use on the skin.
- Eye irritation if EMLA cream accidentally gets into the eye during use on the skin.
Frequency not known(cannot be estimated from the available data):
- Chemical burns to the eyes if EMLA cream accidentally gets into the eye during treatment.
Additional side effects in children
Methemoglobinemia, a blood disorder, which is more commonly observed in children, often in association with overdose in newborns and infants from 0 to 12 months old.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store EMLA
The medicine should be stored out of sight and reach of children.
Do not freeze. Store the tube tightly closed.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What EMLA contains
- The active substances of EMLA are lidocaine and prilocaine. 1 g of cream contains 25 mg of lidocaine and 25 mg of prilocaine.
- EMLA also contains macrogolglycerol hydroxystearate, carbomers, sodium hydroxide, and purified water.
What EMLA looks like and what the packaging contains
White, homogeneous cream.
EMLA is packaged in an aluminum tube with a membrane coated with a protective varnish based on epoxy resin, with a polypropylene cap and a piercer, in a cardboard box.
EMLA is available in the following packaging:
1 tube containing 30 g of cream in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Spain, the country of export:
Aspen Pharma Trading Limited
3016 Lake Drive
Citywest Business Campus
Dublin 24, Ireland
Manufacturer:
Recipharm Karlskoga AB
Björkbornsvägen 5
S-69133 – Karlskoga
Sweden
Aspen Bad Oldesloe GmbH
32-36 Industriestrasse
23843 Bad Oldesloe
Germany
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Spanish marketing authorization number:679290.2
Parallel import authorization number:2/20
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Emla 5% - Creme
Belgium
Emla 25mg/25mg crème
Cyprus
Emla Cream 5%
Finland
EMLA
France
EMLA 5 POUR CENT, crème
Greece
EMLA
Iceland
Emla
Ireland
EMLA 5% w/w Cream
Italy
EMLA
Luxembourg
Emla 25mg/25mg crème
Malta
EMLA 5% w/w Cream
Norway
Emla
Poland
EMLA
Spain
EMLA 25 mg/g + 25 mg/g crema
Sweden
EMLA
Netherlands
Emla
Date of approval of the leaflet: 12.12.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Aspen Pharma Trading Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EmlaDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not required
Alternatives to Emla in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Emla in Spain
Alternative to Emla in Ukraine
Online doctors for Emla
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Emla – subject to medical assessment and local rules.














