

Emla Plaster

Ask a doctor about a prescription for Emla Plaster

How to use Emla Plaster
Leaflet attached to the packaging: information for the user
Warning! Keep the leaflet, information on the immediate packaging in a foreign language!
EMLA PLASTER(Emla Penso)
25 mg + 25 mg, medicinal plaster
Lidocaine + Prilocaine
EMLA PLASTER and Emla Penso are different trade names for the same medicine.
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor, pharmacist, or nurse.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or more information, you should talk to your pharmacist.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in the leaflet. See section 4.
Table of contents of the leaflet
- 1. What is EMLA PLASTER and what is it used for
- 2. Important information before using EMLA PLASTER
- 3. How to use EMLA PLASTER
- 4. Possible side effects
- 5. How to store EMLA PLASTER
- 6. Contents of the pack and other information
1. What is EMLA PLASTER and what is it used for
EMLA PLASTER contains two active substances – lidocaine and prilocaine. They belong to a group of medicines called local anesthetics.
The action of EMLA PLASTER is to temporarily numb the sensation in the superficial layers of the skin. The medicine is applied to the skin before certain medical procedures and treatments. This helps to numb the pain in the skin; however, the patient may still feel pressure and touch.
Adults, adolescents, and children
EMLA PLASTER can be used to numb the skin before:
- injection with a needle (e.g., before giving an injection or taking blood for tests)
- minor surgical procedures on the skin.
2. Important information before using EMLA PLASTER
When not to use EMLA PLASTER:
- if you are allergic to lidocaine, prilocaine, or any other similar local anesthetic or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use EMLA PLASTER, you should discuss it with your doctor or pharmacist:
- if you have a rare metabolic disorder that affects the blood and is called "glucose-6-phosphate dehydrogenase deficiency",
- if you have a problem related to the concentration of a pigment in the blood, called "methemoglobinemia",
- you should not use EMLA PLASTER on areas of skin with a rash, cuts, scratches, or other open wounds. If you have any of these skin conditions, you should contact your doctor or pharmacist before using the plaster,
- if you have a skin disorder called "atopic eczema", it may be sufficient to use the plaster for a shorter time. Using the plaster for more than 30 minutes is associated with a higher risk of a local skin reaction (see also section 4 "Possible side effects").
You should avoid contact between EMLA PLASTER and your eyes, as it may cause irritation. If EMLA PLASTER accidentally gets into your eye, you should immediately rinse your eye with lukewarm water or a physiological saline solution (0.9% NaCl solution).
You should be careful not to get anything in your eye until feeling returns.
If EMLA PLASTER is used before administering a live vaccine (e.g., tuberculosis vaccine), you should remember to return for a follow-up visit at the time specified by your doctor to assess the effectiveness of the vaccination.
Children and adolescents
In infants and newborns under 3 months of age, a transient, clinically insignificant increase in methemoglobin concentration in the blood (a form of hemoglobin, or blood pigment) is commonly observed during the 12 hours following the application of EMLA PLASTER.
The effectiveness of EMLA PLASTER during blood sampling from the heel in newborns has not been confirmed in clinical trials.
EMLA PLASTER should not be used in children under 12 months of age who are being treated with other medicines that may cause methemoglobinemia (e.g., sulfonamides; see also section 2 "EMLA PLASTER and other medicines").
EMLA PLASTER should not be used in premature newborns.
EMLA PLASTER and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take. This includes medicines that can be bought without a prescription and herbal medicines. This is important because the ingredients of EMLA PLASTER may affect the action of some other medicines or some other medicines may affect the action of EMLA PLASTER.
In particular, you should inform your doctor, pharmacist, or nurse if you have used or taken any of the following medicines:
- medicines used to treat infections called sulfonamides and nitrofurantoin
- medicines used to treat epilepsy: phenytoin and phenobarbital
- other local anesthetics
- cimetidine or beta-adrenergic blockers, which may increase the concentration of lidocaine in the blood. This interaction is not clinically significant in short-term use of EMLA PLASTER in recommended doses.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, you should ask your doctor or pharmacist for advice before using this medicine.
Occasional use of EMLA PLASTER during pregnancy is not associated with any increased risk of side effects in the fetus.
The active substances of EMLA PLASTER (lidocaine and prilocaine) pass into breast milk.
However, the amount that passes into breast milk is so small that there is essentially no risk to the baby being breastfed.
Animal studies have not shown any fertility problems in males or females treated with the active substances of EMLA PLASTER.
Driving and using machines
EMLA PLASTER has no or negligible influence on the ability to drive and use machines when used as recommended.
EMLA PLASTER contains macrogolglycerol hydroxystearate
The medicine may cause skin reactions.
3. How to use EMLA PLASTER
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor, pharmacist, or nurse. If you are unsure, you should talk to your doctor, pharmacist, or nurse.
Using EMLA PLASTER
- The site of application of the plasters, the number of plasters, and the duration of application depend on the purpose of use.
- Your doctor, pharmacist, or nurse will apply the plaster to the appropriate area or show you how to do it yourself.
Do not use EMLA PLASTER on the following areas:
- Cuts, scratches, or wounds.
- Areas with a skin rash or eczema.
- Near the eyes.
- Inside the mouth.
Using on the skin before minor procedures (e.g., needle insertion or minor surgical procedures on the skin):
- The plaster is stuck to the skin. Your doctor, pharmacist, or nurse will tell you where to stick the plaster.
- The plaster is removed immediately before starting the procedure.
- Usually, the dose used in adults and adolescents over 12 years is one or more plasters.
- In adults and adolescents over 12 years, the plaster should be applied at least 60 minutes before the planned time of the procedure. However, the plaster should not be applied 5 hours before the procedure or earlier.
- In children, the number of EMLA PLASTER plasters used and the duration of application depend on the child's age. Your doctor, nurse, or pharmacist will inform you how many plasters to use and when to apply them.
Using in children:
Using on the skin before minor procedures (e.g., needle insertion or minor
surgical procedures on the skin):Application time: approximately 1 hour.
Newborns and infants from 0 to 2 months:One plaster is applied to the selected area of skin. Application time: 1 hour, no longer. Only one single dose should be
used in any 24-hour period. The size of the plaster makes it less suitable for use on some parts of the body in newborns and infants.
Infants from 3 to 11 months:Up to 2 plasters are applied to the selected area of skin. Application time: approximately 1 hour.
Children from 1 to 5 years:Up to 10 plasters are applied to the selected area of skin. Application time: approximately 1 hour, maximum 5 hours.
Children from 6 to 11 years:Up to 20 plasters are applied to the selected area of skin. Application time: approximately 1 hour, maximum 5 hours.
In children over 3 months, a maximum of 2 doses (as described above) can be used in any 24-hour period, with an interval of at least 12 hours between doses.
EMLA PLASTER can be used in children with a skin condition called "atopic eczema", but the application time should not exceed 30 minutes in this case.
When using the plaster, it is essential to follow the instructions below carefully:
EMLA PLASTER should be applied at least 1 hour before the procedure (except for patients with atopic eczema, see "Warnings and precautions"). If necessary, before applying the plaster, you should remove any hair from the area of skin. The plaster should not be cut or divided into smaller pieces.
- 1. You should make sure the skin surface to be numbed is clean and dry. You should grasp one of the corners of the protective aluminum foil and fold it back. Then, you should grasp the light beige-colored layer of the plaster at the same corner. Before proceeding, you should make sure the two layers in the corner of the plaster have been properly separated.
- 2. Pull and separate the two layers from each other, thus separating the adhesive surface from the protective foil, as shown in the diagram. You should be careful not to touch the round white disc, which contains the medicine.
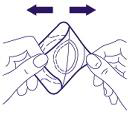
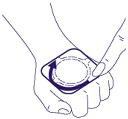
- 3. You should not press the central part of the plaster. Pressing this part of the plaster may cause the medicine to leak under the adhesive layer and prevent the plaster from sticking properly to the skin. You should press the edges of the plaster firmly to ensure good adhesion to the skin.

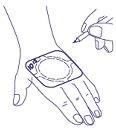
- 4. The time of application of the plaster to the skin can be written directly on the edge of the plaster. (A pen can be used for this purpose.)
- 5. Leave the plaster on for at least one hour (except for patients with atopic eczema, see "Warnings and precautions"). In children under 3 months, the plaster should not be left on for more than 1 hour.
- 6. After the application time has passed, you should remove the plaster from the skin.
Using on the skin before removing warts
- EMLA PLASTER can be used in children and adolescents with a skin condition called "atopic eczema".
- The recommended dose depends on the child's age and is applied for 30 to 60 minutes (30 minutes if the patient has atopic eczema). Your doctor, nurse, or pharmacist will tell you how many plasters to use.
Using more EMLA PLASTER than recommended
If you use more EMLA PLASTER than described in the leaflet or recommended by your doctor, pharmacist, or nurse, you should tell them immediately, even if you do not notice any side effects.
The symptoms that may occur after using too much EMLA PLASTER are listed below. These symptoms should not occur when using EMLA PLASTER as recommended.
- A feeling of "emptiness" in the head or dizziness.
- Numbness or tingling of the skin around the mouth and tongue.
- Disturbances of taste.
- Blurred vision.
- Ringing in the ears.
- There is also a risk of acute methemoglobinemia (a problem with the concentration of a pigment in the blood). The risk of this is higher if you are taking certain other medicines. If this occurs, the skin becomes blue-gray due to insufficient oxygen in the blood.
In severe cases of overdose, the following symptoms may occur: seizures, decreased blood pressure, slow breathing, respiratory arrest, and abnormal heart rhythm. These symptoms can be life-threatening.
If you have any further questions about using this medicine, you should talk to your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, EMLA PLASTER can cause side effects, although not everybody gets them.
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in the leaflet.
At the site of application of EMLA PLASTER, a mild reaction may occur (pallor or redness of the skin, slight swelling, initial burning or itching sensation). These are normal reactions to the plaster and anesthetics, which disappear after a short time without the need for any medical treatment.
If you get any worrying or unusual side effects or reactions while using EMLA PLASTER, you should stop using it and talk to your doctor or pharmacist immediately.
Common(may affect up to 1 in 10 people):
- Transient local skin reactions (pallor, redness, swelling) at the site of application during use on the skin.
Uncommon(may affect up to 1 in 100 people):
- Initial mild burning, itching, or warm sensation at the site of application during use on the skin.
Rare(may affect up to 1 in 1,000 people):
- Allergic reactions, which in rare cases may lead to anaphylactic shock (skin rash, swelling, fever, difficulty breathing, and fainting).
- Methemoglobinemia (a blood disorder).
- Minor pinpoint bleeding (petechiae) at the site of application (especially in children with eczema after prolonged use of the medicine).
- Irritation of the eyes if EMLA PLASTER accidentally comes into contact with them during use on the skin.
Additional side effects in children
Methemoglobinemia, a blood disorder, which is more commonly observed in children, often in association with overdose in newborns and infants under 12 months of age.
Reporting side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in the leaflet. You can also report side effects directly to the national reporting system (see below for contact information).
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store EMLA PLASTER
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging. The expiry date refers to the last day of that month.
Do not store above 30°C. Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What EMLA PLASTER contains
- The active substances are lidocaine and prilocaine. One medicinal plaster of approximately 10 cm² contains 25 mg of lidocaine and 25 mg of prilocaine.
- The other ingredients are: macrogolglycerol hydroxystearate, carbomer, sodium hydroxide, purified water.
What EMLA PLASTER looks like and contents of the pack
EMLA PLASTER consists of a part to be stuck to the skin and a protective foil. The part to be stuck to the skin is light beige in color. In the center of the plaster, there is a round white disc containing the active substances and excipients. The rest of the plaster is covered with an acrylic adhesive.
Pack sizes:
2 plasters, protected on one side by aluminum foil, in a cardboard box.
For more detailed information, you should talk to the marketing authorization holder or the parallel importer.
Marketing authorization holder in Portugal, the country of export:
Aspen Pharma Trading Limited
3016 Lake Drive
Citywest Business Campus
Dublin 24
Ireland
Manufacturer:
Recipharm Karlskoga AB
Björkbornvägen, 5
SE-691 33 Karlskoga
Sweden
Parallel importer:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
Repackaged by:
Medezin Sp. z o.o.
ul. Zbąszyńska 3
91-342 Łódź
Marketing authorization number in Portugal, the country of export: 2426385
2426286
Parallel import authorization number: 72/24
Date of revision of the leaflet: 23.02.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Aspen Pharma Trading Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Emla PlasterDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Cream, 25 mg/g + 25 mg/gActive substance: combinationsPrescription required
Alternatives to Emla Plaster in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Emla Plaster in Spain
Alternative to Emla Plaster in Ukraine
Online doctors for Emla Plaster
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Emla Plaster – subject to medical assessment and local rules.














