

Emla Plaster

Ask a doctor about a prescription for Emla Plaster

How to use Emla Plaster
Leaflet attached to the packaging: information for the user
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
EMLA PLASTER (EMLA), 25 mg + 25 mg, medicinal plaster
Lidocaine + Prilocaine
EMLA PLASTER and EMLA are different trade names for the same medicine.
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor, pharmacist, or nurse.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or more information, you should speak to a pharmacist.
- If you experience any side effects, including any not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is EMLA PLASTER and what is it used for
- 2. Important information before using EMLA PLASTER
- 3. How to use EMLA PLASTER
- 4. Possible side effects
- 5. How to store EMLA PLASTER
- 6. Contents of the packaging and other information
1. What is EMLA PLASTER and what is it used for
EMLA PLASTER contains two active substances - lidocaine and prilocaine. They belong to a group of medicines called local anesthetics.
The action of EMLA PLASTER is to temporarily numb the sensation in the superficial layers of the skin. The medicine is applied to the skin before certain medical procedures. This helps to numb the pain in the skin; however, the patient may still feel pressure and touch.
Adults, adolescents, and children
EMLA PLASTER can be used to numb the skin before:
- injection with a needle (e.g., before receiving an injection or having blood drawn for tests)
- minor surgical procedures on the skin.
2. Important information before using EMLA PLASTER
When not to use EMLA PLASTER:
Warnings and precautions
Before starting to use EMLA PLASTER, you should discuss it with your doctor or pharmacist:
- you should not use EMLA PLASTER on areas of skin with a rash, cuts, scrapes, or other open wounds. If you have any of these conditions, you should contact your doctor or pharmacist before using the plaster,
which may require a shorter application time. Using the plaster for more than 30 minutes is associated with a higher probability of a local skin reaction (see also section 4 "Possible side effects").
You should avoid contact between EMLA PLASTER and your eyes, as it may cause irritation. If EMLA PLASTER accidentally gets into your eye, you should immediately rinse your eye with lukewarm water or a physiological saline solution (0.9% NaCl solution).
You should be careful not to get anything in your eye until the feeling returns.
If EMLA PLASTER is used before administering a live vaccine (e.g., tuberculosis vaccine), you should remember to return for a follow-up visit at the time specified by your doctor to assess the effectiveness of the vaccination.
Children and adolescents
In infants and newborns under 3 months of age, a transient, clinically insignificant increase in methemoglobin levels in the blood is commonly observed within 12 hours of applying EMLA PLASTER.
The effectiveness of EMLA PLASTER during blood sampling from the heel in newborns has not been confirmed in clinical trials.
EMLA PLASTER should not be used in children under 12 months of age who are being treated with other medicines that may cause methemoglobinemia (e.g., sulfonamides; see also section 2 "EMLA PLASTER and other medicines").
EMLA PLASTER should not be used in premature newborns.
EMLA PLASTER and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take. This includes medicines that can be bought without a prescription and herbal medicines. This is important because the ingredients of EMLA PLASTER may affect the action of some other medicines or other medicines may affect the action of EMLA PLASTER.
In particular, you should inform your doctor, pharmacist, or nurse if you have used or taken any of the following medicines:
- medicines used to treat infections called sulfonamides and nitrofurantoin
- medicines used to treat epilepsy: phenytoin and phenobarbital
- other local anesthetics
- cimetidine or beta-adrenergic blockers, which may increase the level of lidocaine in the blood. This interaction is not clinically significant in short-term use of EMLA PLASTER in recommended doses.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Occasional use of EMLA PLASTER during pregnancy is not associated with any increased risk of side effects in the fetus.
The active substances of EMLA PLASTER (lidocaine and prilocaine) pass into breast milk.
However, the amount that passes into breast milk is so small that there is essentially no risk to the breastfed child.
Animal studies have not shown any fertility problems in males or females treated with the active substances of EMLA PLASTER.
Driving and using machines
EMLA PLASTER does not affect or has negligible effects on the ability to drive and use machines when used as recommended.
EMLA PLASTER contains macrogolglycerol hydroxystearate
The medicine may cause skin reactions.
3. How to use EMLA PLASTER
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor, pharmacist, or nurse. If you are unsure, you should speak to your doctor, pharmacist, or nurse.
Using EMLA PLASTER
- The site of application, the number of plasters, and the application time depend on the purpose of use.
- Your doctor, pharmacist, or nurse will apply the plaster to the appropriate area or show you how to do it yourself.
Do not use EMLA PLASTER on the following areas:
- Cuts, scrapes, or wounds.
- Areas with a skin rash or eczema.
- Near the eyes.
- Inside the mouth.
Using on the skin before minor procedures (e.g., needle injection or minor surgical procedures on the skin):
- The plaster is applied to the skin. Your doctor, pharmacist, or nurse will tell you where to apply the plaster.
- The plaster is removed immediately before the procedure starts.
- Usually, the recommended dose for adults and adolescents over 12 years is one or more plasters.
- For adults and adolescents over 12 years, the plaster should be applied at least 60 minutes before the planned procedure time. However, the plaster should not be applied 5 hours before the procedure or earlier.
- For children, the number of EMLA PLASTER plasters used and the application time depend on the child's age. Your doctor, nurse, or pharmacist will inform you how many plasters to use and when to apply them.
Using in children:
Using on the skin before minor procedures (e.g., needle injection or minor
skin procedures):Application time: approximately 1 hour.
Newborns and infants from 0 to 2 months:One plaster is applied to the selected skin area. Application time: 1 hour, no longer. Only one single dose should be
used in any 24-hour period.The size of the plaster makes it less suitable for use on some parts of the body in newborns and infants.
Infants from 3 to 11 months:Up to 2 plasters are applied to the selected skin area. Application time: approximately 1 hour.
Children from 1 to 5 years:Up to 10 plasters are applied to the selected skin area. Application time: approximately 1 hour, maximum 5 hours.
Children from 6 to 11 years:Up to 20 plasters are applied to the selected skin area. Application time: approximately 1 hour, maximum 5 hours.
In children over 3 months, a maximum of 2 doses (as described above) can be used in any 24-hour period, with an interval of at least 12 hours between doses.
EMLA PLASTER can be used in children with a skin condition called "atopic dermatitis", but the application time should not exceed 30 minutes.
When using the plaster, it is essential to follow the instructions below carefully:
EMLA PLASTER should be applied at least 1 hour before the procedure (except for patients with atopic dermatitis, see "Warnings and precautions"). If necessary, hair should be removed from the skin area before applying the plaster. The plaster should not be cut or divided into smaller pieces.
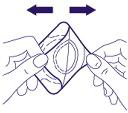
- 1. Make sure the skin area to be anesthetized is clean and dry. Hold one of the corners of the aluminum foil protecting the plaster and fold it back. Then, hold the light beige-colored layer of the plaster by the same corner. Before proceeding, make sure the two layers in the corner of the plaster have been properly separated.
- 2. Pull and separate the two layers from each other, thus separating the adhesive surface from the protective foil, according to the diagram. Be careful not to touch the round white disk that contains the medicine.
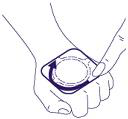
- 3. Do not press the central part of the plaster. Pressing this part of the plaster may cause the medicine to leak under the adhesive layer and prevent the plaster from sticking properly to the skin. Press the edges of the plaster firmly to ensure good adhesion to the skin.
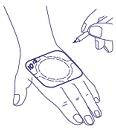
- 4. The time of application can be written directly on the edge of the plaster. (A ballpoint pen can be used for this purpose.)
- 5. Leave the plaster on for at least 1 hour (except for patients with atopic dermatitis, see "Warnings and precautions"). In children under 3 months, the plaster should not be left on for more than 1 hour.

- 6. After the application time has passed, remove the plaster from the skin.
Using on the skin before removing warts
- EMLA PLASTER can be used in children and adolescents with a skin condition called "atopic dermatitis".
- The recommended dose depends on the child's age and is applied for 30 to 60 minutes (30 minutes if the patient has atopic dermatitis). Your doctor, nurse, or pharmacist will tell you how many plasters to use.
Using more EMLA PLASTER than recommended
If you use more EMLA PLASTER than described in the leaflet or recommended by your doctor, pharmacist, or nurse, you should immediately inform them, even if you do not notice any side effects.
Symptoms that may occur after using too much EMLA PLASTER are listed below. These symptoms should not occur when using EMLA PLASTER as recommended.
- Feeling "empty-headed" or dizzy.
- Numbness or tingling of the skin around the mouth and tongue.
- Abnormal taste.
- Blurred vision.
- Ringing in the ears.
- There is also a risk of acute methemoglobinemia (a problem with the concentration of a blood pigment). The risk of this is higher if you are taking certain other medicines. If this occurs, the skin becomes blue-gray due to insufficient oxygen in the blood.
In severe cases of overdose, the following symptoms may occur: seizures, low blood pressure, slow breathing, respiratory arrest, and abnormal heart rhythm. These symptoms can be life-threatening.
If you have any further questions about using this medicine, you should speak to your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, EMLA PLASTER can cause side effects, although not everybody gets them.
If you experience any of the following side effects, you should contact your doctor or pharmacist immediately. You should tell your doctor about everything that makes you feel unwell when using EMLA PLASTER.
At the site of application of EMLA PLASTER, a mild reaction may occur (pallor or redness of the skin, slight swelling, initial burning or itching sensation). These are normal reactions to the plaster and anesthetics, which disappear after a short time without the need for any medical treatment.
If you experience any worrying or unusual effects or reactions when using EMLA PLASTER, you should stop using it and contact your doctor or pharmacist immediately.
Common(may affect up to 1 in 10 people):
- Transient local skin reactions (pallor, redness, swelling) at the site of application during use on the skin.
Uncommon(may affect up to 1 in 100 people):
- Initial mild burning, itching, or warm sensation at the site of application during use on the skin.
Rare(may affect up to 1 in 1,000 people):
- Allergic reactions, which in rare cases can lead to anaphylactic shock (skin rash, swelling, fever, difficulty breathing, and fainting).
- Methemoglobinemia (a blood disorder).
- Minor pinpoint bleeding (petechiae) at the site of application (especially in children with eczema after prolonged use of the medicine).
- Irritation of the eyes if EMLA PLASTER accidentally comes into contact with them during use on the skin.
Additional side effects in children
Methemoglobinemia, a blood disorder, which is more commonly observed in children, often in association with overdose in newborns and infants under 12 months of age.
Reporting side effects
If you experience any side effects, including any not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 22 49 21 301
fax: + 48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store EMLA PLASTER
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Store in a temperature below 25°C. Do not store in the refrigerator or freeze.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What EMLA PLASTER contains
- The active substances are lidocaine and prilocaine. One medicinal plaster with an area of approximately 10 cm² contains 25 mg of lidocaine and 25 mg of prilocaine.
- The other ingredients are: macrogolglycerol hydroxystearate (arlatone 289), carbomer, sodium hydroxide, purified water.
What EMLA PLASTER looks like and what the pack contains
EMLA PLASTER consists of a part that sticks to the skin and a protective foil. The part that sticks to the skin is light beige in color. In the center of the plaster, there is a round white disk containing the active substances and excipients. The rest of the plaster is covered with an acrylic adhesive.
Pack size
2 plasters, protected on one side by aluminum foil, in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Greece, the country of export:
Aspen Pharma Trading Limited, 3016 Lake Drive, Citywest Business Campus, Dublin 24, Ireland
Manufacturer:
Recipharm Karlskoga AB, Bjorkbornsvagen 5, 691 33, Karlskoga, Sweden
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Marketing authorization number in Greece, the country of export: 78818/16/22-03-17
Parallel import authorization number: 151/24
Date of approval of the leaflet: 11.04.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Aspen Pharma Trading Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Emla PlasterDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Plaster, 25 mg + 25 mgActive substance: combinationsPrescription not requiredDosage form: Cream, 25 mg/g + 25 mg/gActive substance: combinationsPrescription required
Alternatives to Emla Plaster in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Emla Plaster in Espanha
Alternative to Emla Plaster in Ukraine
Online doctors for Emla Plaster
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Emla Plaster – subject to medical assessment and local rules.














