
ORAQIX GEL PERIODONTAL
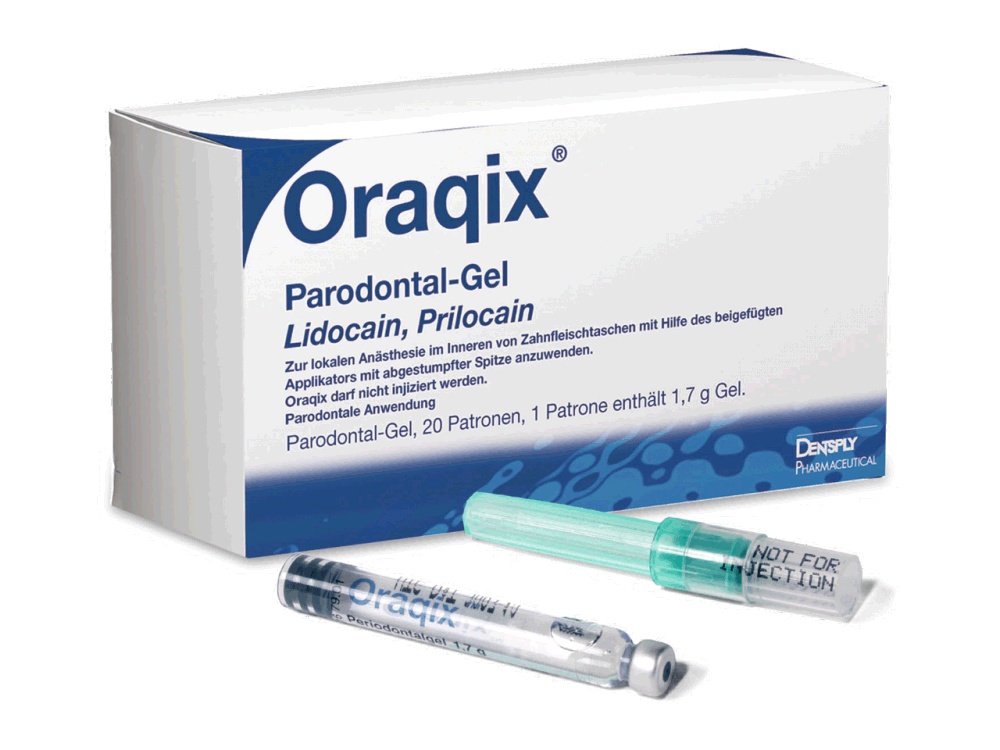

How to use ORAQIX GEL PERIODONTAL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Oraqix Periodontal Gel
Lidocaine and Prilocaine
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your dentist or dental hygienist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any of the side effects, talk to your dentist, dental hygienist, or doctor. This includes any possible side effects not listed in this leaflet.
Contents of the pack:
- What Oraqix is and what it is used for
- Before using Oraqix
- How to use Oraqix
- Possible side effects
- Storage of Oraqix
- Further information
1. What Oraqix is and what it is used for
Oraqix is a gel used to prevent gum pain. Oraqix is used in adults to control pain during certain types of dental procedures, such as root planing and scaling (cleaning parts of your teeth that your toothbrush cannot reach) and probing (examining your teeth and gums).
2. Before using Oraqix
Do not use Oraqix
- if you are allergic (hypersensitive) to lidocaine, prilocaine, or other similar local anesthetics;
- if you are allergic (hypersensitive) to any of the other ingredients of the gel (see section 6, Further information);
- if your doctor has told you that you have congenital or idiopathic methemoglobinemia (a disorder in which too much of the hemoglobin in your blood is converted to methemoglobin. If too much methemoglobin is formed, it becomes more difficult for the blood to provide oxygen to the tissue);
- if your doctor has told you that you suffer from repeated episodes of porphyria or if you may have inherited porphyria (porphyrias are a group of disorders that affect the way your blood is produced).
Talk to your dentist or dental hygienist if you are in any of the following situations:
- if you have kidney or liver disease;
- if you have irregular heart activity or circulation problems;
- if you have ulcers or an infection in the mouth;
- if your doctor has told you that you have a deficiency of the enzyme glucose-6-phosphate (a hereditary condition that occurs when the body lacks the important enzyme glucose-6-phosphate).
Be careful with Oraqix
- The use of Oraqix in children and adolescents has not been evaluated and therefore its use is not recommended in persons under 18 years of age.
- Avoid getting Oraqix in your eyes. If the gel accidentally gets into your eyes, rinse them with water or saline solution immediatelyand protect them until sensitivity returns.
- Oraqix may occasionally block all sensitivity in the treated areas. Try not to bite your mouth inadvertently. Also, avoid taking very hot drinks and food until you have fully regained sensitivity.
- Oraqix may interfere with tests for banned substances in athletes. It could give a false positive result for these substances.
Using other medicines
Tell your dentist if you are using:
- any other local anesthetic of the amide type or products used to treat irregular heart activity (antiarrhythmics such as mexiletine), as these medicines in combination with Oraqix may increase the risk of the side effects described in the section "If you use more Oraqix than you should"
- other medicines that may cause methemoglobinemia, such as certain types of antibiotics known as sulfonamides.
Tell your dentist or dental hygienist if you are using or have recently used other medicines, including those obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant, planning to become pregnant, or breastfeeding, consult your dentist or dental hygienist before taking Oraqix. Consult your doctor or pharmacist before using any medicine.
- Oraqix should notbe used during pregnancy unless your dentist recommends it.
- Breastfeeding can continue after treatment with Oraqix.
Driving and using machines
Oraqix has no known effects that could affect your ability to drive or use machines.
3. How to use Oraqix
Oraqix will be administered by a dentist or dental hygienist. The dose is decided by the dentist or dental hygienist and depends on how many teeth need to be treated.
- Oraqix periodontal gel is intended for use in adults.
- Oraqix must not be injected.
- Oraqix gel is applied inside the gum with a dental syringe or using the Oraqix dispenser and a blunt-tipped applicator.
- The maximum dose in a single treatment is 5 cartridges.
- The full effect is achieved after about half a minute, and then the dentist or dental hygienist can start the treatment. The effect should last about 20 minutes.
- If you feel that the effect of Oraqix is too strong or too weak, tell your dentist or dental hygienist.
- You may receive other local anesthetics during the same treatment session.
- Frequent use of large amounts of Oraqix is not recommended.
If you use more Oraqix than you should
If you are given too much local anesthesia (i.e., Oraqix in combination with another anesthetic), the following side effects may appear: numbness of the lips and around the mouth, nervousness, dizziness, vertigo, agitation, or sometimes blurred vision, numbness, and loss of consciousness. Other rare effects are convulsions, difficulty breathing, and low blood pressure.
An overdose of prilocaine (Oraqix in combination with another anesthetic containing prilocaine) can also cause methemoglobinemia (see section 2). This is characterized by a grayish cyanosis (a bluish-gray discoloration of the lips and skin).
If you experience any of these symptoms, consult your dentist, dental hygienist, or doctor, and go to the emergency department of the nearest hospital for risk assessment and advice. You may need to be kept under observation for a few hours.
4. Possible side effects
Like all medicines, Oraqix can cause side effects, although not everybody gets them.
Common side effects (affecting up to 1 in 10 people):
- headache
- changes in taste
- reactions in the mouth (pain, burning, numbness of other parts of the mouth or lips, ulcer, irritation, and redness)
Uncommon side effects (affecting less than 1 in 100 people):
- allergic reactions that could include: skin rash, swelling of the throat, difficulty breathing, and fever
- dizziness
- nausea
- breathing difficulties and cyanosis (a bluish-gray discoloration of the lips and skin)
- burning sensation
- swelling at the site of application
- An overdose of Oraqix could lead to excitement or depression of the central nervous system and depression of cardiac circulation and heart function. In case of any concern regarding these possible side effects, go directly to the hospital.
If any side effect gets worse, for example, you have difficulty breathing or swelling of the throat, or if an anaphylactic reaction to the anesthetic is suspected, tell your dentist or a doctor immediately or go directly to the hospital. If you experience any side effect that is severe or if you notice any side effect not listed in this leaflet, tell your dentist or dental hygienist if you are still at the clinic or your doctor if you are already at home when they occur.
5. Storage of Oraqix
- Keep out of the reach and sight of children.
- Do not freeze.
- Do not use Oraqix after the expiry date stated on the packaging. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Further information
Composition of Oraqix
The active substances are prilocaine (base) and lidocaine (base).
The other ingredients are purified poloxamer 188, purified poloxamer 407, purified water, and diluted hydrochloric acid, used to adjust the pH.
Appearance of Oraqix and packaging contents
Oraqix is a periodontal gel. It is a clear, colorless gel in a glass cartridge and is for single use.
Each gram of Oraqix Periodontal Gel contains 25 mg of lidocaine and 25 mg of prilocaine.
Each cartridge contains 1.7 g of gel (42.5 mg of lidocaine and 42.5 mg of prilocaine).
Each pack contains 20 cartridges.
Marketing authorization holder
DENTSPLY DETREY GmbH, De-Trey-Str. 1, 78467 Konstanz, Germany
Phone: +49-(0)7531-583-0
Manufacturer
Recipharm Karlskoga AB, Björkbornsvägen 5, SE-691 33 Karlskoga Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
MEMBER STATE | NAME OF THE MEDICINAL PRODUCT |
Austria | Oraqix Parodontal-Gel Lidocaine and Prilocaine |
Denmark | Oraqix periodontalgel Lidocaine and Prilocaine |
Finland | Oraqix geeli ientaskuun Lidocaine and Prilocaine |
Germany | Oraqix Parodontal-Gel Lidocaine and Prilocaine |
Iceland | Oraqix tannholdshlaup Lidocaine and Prilocaine |
Netherlands | Oraqix periodontale gel Lidocaine and Prilocaine |
Norway | Oraqix periodontalgel Lidocaine and Prilocaine |
Sweden | Oraqix periodontalgel Lidocaine and Prilocaine |
Belgium | Oraqix 25/25 mg Gel periodontal Lidocaine and Prilocaine |
Luxembourg | Oraqix 25/25 mg Gel periodontal Lidocaine and Prilocaine |
Ireland | Oraqix 25/25 mg per g Periodontal Gel Lidocaine and Prilocaine |
United Kingdom | Oraqix 25/25 mg per g Periodontal Gel Lidocaine and Prilocaine |
France | Oraqix gel périodontal Lidocaine and Prilocaine |
Portugal | Oraqix Gel Periodontal Lidocaine and Prilocaine |
Spain | Oraqix Gel Periodontal Lidocaine and Prilocaine |
Italy | Oraqix 25/25 mg/g Gel Periodontale Lidocaine and Prilocaine |
This leaflet was approved on 23 June 2010.
Cut
…………………………………………………………………………………………
The following information is intended for medical professionals only:
For more information on the use of Oraqix, consult the Summary of Product Characteristics (SPC).
Method of administration
- Oraqix must not be injected
- On average, one cartridge (1.7 g) or less of Oraqix will be sufficient for one quadrant of the dentition. The maximum recommended dose of Oraqix in a treatment session is five cartridges, i.e., 8.5 g of gel containing 212.5 mg of lidocaine base and 212.5 mg of prilocaine base.
- At the time of administration, Oraqix should be a liquid. If a gel has formed, it should be placed in the refrigerator until it returns to a liquid consistency. The visible air bubble in the cartridge will move when tilted.
- The gel may become opaque at temperatures below 5°C. The gel will regain its original appearance when it reaches room temperature. Oraqix becomes a gel after administration.
- The cartridge and applicator are intended for single use. They fit into the Oraqix dispenser.
- Fill the periodontal pockets with Oraqix gel using a dental syringe or the Oraqix dispenser and the blunt-tipped applicator provided with the package, until the gel appears at the gingival margin. Wait half a minute before starting treatment (a longer waiting time will not increase the anesthesia). The duration of anesthesia, evaluated by probing the pocket depth, is about 20 minutes. If the anesthesia starts to decrease, reapply Oraqix as needed.
- If you need additional local anesthesia in combination with Oraqix, consult the SPC for each additional anesthetic. Since systemic toxic effects are additive (see sections 4.5 and 4.9 of the Oraqix SPC), it is not recommended to give more local anesthetics during the same treatment session if the amount of Oraqix administered corresponds to the maximum recommended dose of five cartridges.
- Avoid contact with Oraqix to prevent the development of a possible allergy.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ORAQIX GEL PERIODONTALDosage form: CREAM, 25 mg/g + 25 mg/gActive substance: combinationsManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: CREAM, 25 mg/g + 25 mg/gActive substance: combinationsManufacturer: Galenicum Derma S.L.U.Prescription requiredDosage form: CREAM, Lidocaine 25 mg/g + Prilocaine 25 mg/gActive substance: combinationsManufacturer: Mesoestetic Pharma Group S.L.Prescription required
Online doctors for ORAQIX GEL PERIODONTAL
Discuss questions about ORAQIX GEL PERIODONTAL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















