BOOSTRIX POLIO Injectable Suspension in Pre-filled Syringe

How to use BOOSTRIX POLIO Injectable Suspension in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Boostrix Polio, Suspension for injection in a pre-filled syringe
Diphtheria, tetanus, pertussis (acellular component) and poliomyelitis (inactivated) (adsorbed, reduced antigen content) vaccine
Read all of this leaflet carefully before you or your child receive this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed to you or your child, do not pass it on to others.
- If you or your child experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Boostrix Polio and what is it used for
- What you need to know before you or your child receive Boostrix Polio
- How to use Boostrix Polio
- Possible side effects
- Storage of Boostrix Polio
- Contents of the pack and other information
1. What is Boostrix Polio and what is it used for
Boostrix Polio is a vaccine indicated for booster vaccination in children from 3 years of age, adolescents and adults to prevent four diseases: diphtheria, tetanus (lockjaw), pertussis and poliomyelitis (polio). The vaccine works by helping the body to produce its own protection (antibodies) against these diseases.
- Diphtheria:Diphtheria mainly affects the respiratory tract and sometimes the skin. The respiratory tract usually becomes inflamed (swollen) causing severe breathing difficulties and sometimes suffocation. The bacteria also release a toxin (poison) that can cause nerve damage, heart problems and even death.
- Tetanus (lockjaw):The tetanus bacteria enter the body through cuts, scratches or wounds in the skin. Wounds that are particularly prone to infection are burns, fractures, deep wounds or wounds contaminated with dirt, dust, horse manure or wood splinters. The bacteria release a toxin (poison) that can cause muscle stiffness, painful muscle spasms, convulsions and even death. The muscle spasms can be so strong that they cause fractures of the spine.
- Pertussis:Pertussis is a highly infectious disease. The disease affects the respiratory tract causing severe coughing attacks that can interfere with normal breathing. The cough is often accompanied by a "whoop" hence the common name whooping cough. The cough can last 1-2 months or longer. It can also cause ear infections, long-lasting bronchitis, pneumonia, convulsions, brain damage and even death.
- Poliomyelitis(polio): Poliomyelitis, sometimes called simply "polio", is a viral infection that can have different effects. It often causes a mild illness but in some people it can cause permanent damage or even death. In its most severe form, polio infection causes paralysis of the muscles (the muscles cannot move), including those muscles necessary for breathing and walking. The affected limbs can become painfully deformed.
None of the components of the vaccine can cause diphtheria, tetanus, pertussis or poliomyelitis.
The use of Boostrix Polio during pregnancy will help protect your baby from pertussis in the first months of life, before they receive their primary immunization.
2. What you need to know before you or your child receive Boostrix Polio
Boostrix Polio must not be administered:
- if you or your child have previously had any allergic reaction to Boostrix Polio or any of the other components of this vaccine (listed in section 6), or to neomycin, polymyxin (antibiotics) or formaldehyde. The signs of an allergic reaction may include skin rash with itching, difficulty breathing and swelling of the face or tongue
- if you or your child have previously had an allergic reaction to any vaccine against diphtheria, tetanus, pertussis or poliomyelitis
- if you or your child have had problems with the nervous system (encephalopathy) in the 7 days following a previous vaccination with a pertussis vaccine
- if you or your child have had a temporary reduction in blood platelets (which increases the risk of bleeding or bruising) or brain or nervous system problems after a previous vaccination with a diphtheria and/or tetanus vaccine
- if you or your child have had a severe infection with a high temperature (over 38°C). A minor infection should not be a problem, but consult your doctor first.
Warnings and precautions
Consult your doctor or pharmacist before you or your child receive Boostrix Polio:
- if you or your child have had any problems after a previous administration of Boostrix Polio or another pertussis vaccine, especially:
- Fever (over 40°C) in the 48 hours following vaccination
- Collapsing or a shock-like state in the 48 hours following vaccination
- Persistent, inconsolable crying lasting more than 3 hours, occurring within 48 hours of vaccination
- Seizures, with or without fever, occurring within 3 days of vaccination
- if your child has an undiagnosed or progressive brain disease or uncontrolled epilepsy. The vaccine should be administered once the disease is under control
- if you or your child have bleeding problems or bruise easily
- if you or your child have a tendency to have febrile seizures or if there is a family history of this
- if you or your child have persistent immune system problems due to any cause (including HIV infection). You or your child may still receive Boostrix Polio but the protection against infections after vaccination may not be as good as in patients with adequate immune responses to infections.
Before or after any injection, fainting (especially in adolescents) may occur, so you should inform your doctor or nurse if you or your child have fainted after a previous injection.
As with all vaccines, Boostrix Polio may not provide complete protection in all vaccinated patients.
Use of Boostrix Polio with other medicines
Tell your doctor or pharmacist if you or your child are using, have recently used or might use any other medicines or if you have recently received any other vaccine.
Boostrix Polio can be administered at the same time as some vaccines. Use a different injection site for each type of vaccine.
Boostrix Polio may not provide an adequate response if you or your child are taking medicines that reduce the effectiveness of your immune system against infections.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before receiving this vaccine.
It is not known whether Boostrix Polio passes into breast milk. Your doctor will inform you of the possible risks and benefits of administering Boostrix Polio during breastfeeding.
Driving and using machines
Boostrix Polio is unlikely to have any effect on the ability to drive or use machines.
Boostrix Polio contains neomycin and polymyxin
This vaccine contains neomycin and polymyxin (antibiotics). Please inform your doctor if you or your child have had an allergic reaction to these components.
Boostrix Polio contains para-aminobenzoic acid, phenylalanine, sodium and potassium
Boostrix Polio contains para-aminobenzoic acid. It may cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm.
This vaccine contains 0.0298 micrograms of phenylalanine per dose. Phenylalanine may be harmful in case of phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
This vaccine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially "sodium-free".
This vaccine contains potassium, less than 1 mmol (39 mg) per dose; this is, essentially "potassium-free".
3. How to use Boostrix Polio
- Boostrix Polio will be administered as an injection into the muscle.
- The vaccine must never be administered intravenously.
- You or your child will receive a single injection of Boostrix Polio.
- Your doctor will check if you or your child have previously received diphtheria, tetanus, pertussis and/or poliomyelitis vaccines.
- Boostrix Polio can be used in case of suspected tetanus infection, although other measures to reduce the risk of disease manifestation, such as wound care and/or administration of tetanus antitoxin, should also be taken.
- Your doctor will recommend repeating the vaccination.
4. Possible side effects
Like all medicines, Boostrix Polio can cause side effects, although not everybody gets them.
As with all injectable vaccines, you or your child may experience severe allergic reactions (anaphylactic and anaphylactoid reactions) in very rare cases (up to a maximum of 1 in 10,000 doses of the vaccine). These can be recognized by:
- Skin rashes such as itching or hives,
- Swelling of the eyes and face,
- Difficulty breathing or swallowing,
- Sudden drop in blood pressure and loss of consciousness.
These reactions can occur before leaving the doctor's office. However, if you notice any of these symptoms in you or your child, you should contact your doctor immediately.
Side effects that have occurred during clinical trials in children aged 4 to 8 years
Very common(may occur with more than 1 in 10 doses of the vaccine): pain, redness and swelling at the injection site, feeling sleepy.
Common(may occur with up to 1 in 10 doses of the vaccine): fever of 37.5°C or higher (including fever over 39°C), bleeding, itching and hardening at the injection site, extensive swelling of the limb where the vaccine was administered, loss of appetite, irritability, headache.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): diarrhea, nausea, vomiting, stomach pain, swelling of the glands in the neck, armpits or groin (lymphadenopathy), sleeping problems, apathy, dry throat, fatigue.
Concomitant administration with measles, mumps, rubella (MMR) or measles, mumps, rubella, varicella (MMRV) vaccines in children aged 3-6 years
In studies where Boostrix Polio was administered at the same time as MMR or MMRV vaccines, skin rashes and upper respiratory tract infections (including runny nose and sore throat) were frequently reported. Fever, irritability, fatigue, loss of appetite and gastrointestinal disorders (including diarrhea and vomiting) were reported more frequently (very common) than in studies where Boostrix Polio was administered alone.
Side effects that have occurred during clinical trials in adults, adolescents and children from 10 years of age:
Very common(may occur with more than 1 in 10 doses of the vaccine): pain, redness and swelling at the injection site, fatigue, headache.
Common(may occur with up to 1 in 10 doses of the vaccine): fever of 37.5°C or higher, bruising, itching, hardening, warmth at the injection site, stomach pain, nausea, vomiting.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): fever over 39°C, extensive swelling of the limb where the vaccine was administered, chills, pain, dizziness, joint pain, muscle pain, itching, oral herpes, swelling of the glands in the neck, armpits or groin (lymphadenopathy), loss of appetite, tingling or numbness of the hands or feet (paresthesia), drowsiness, asthma.
The following side effects have occurred during routine use of Boostrix Polio and are not specific to any age group: fainting or loss of consciousness, swelling of the face, lips, mouth, tongue or throat that can cause difficulty swallowing or breathing (angioedema), seizures or fits (with or without fever), hives (urticaria), unusual weakness (asthenia).
Additionally, the following side effects were reported during clinical trials with Boostrix (GlaxoSmithKline's diphtheria, tetanus and pertussis booster vaccine):
Side effects that have occurred in children aged 4 to 8 years
Uncommon(may occur with up to 1 in 100 doses of the vaccine): attention disorders, itchy eyes and eyelids with crust (conjunctivitis), pain.
Side effects that have occurred in adults, adolescents and children from 10 years of age:
Very common(may occur with more than 1 in 10 doses of the vaccine): general feeling of being unwell.
Common(may occur with up to 1 in 10 doses of the vaccine): induration or abscess at the injection site.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): upper respiratory tract infection, sore throat and difficulty swallowing (pharyngitis), fainting (syncope), cough, diarrhea, excessive sweating (hyperhidrosis), skin rash, joint stiffness, muscle stiffness and joint stiffness, flu-like symptoms such as fever, sore throat, runny nose, cough and chills.
After administration of tetanus vaccines, in very rare cases (up to a maximum of 1 in 10,000 doses of the vaccine), cases of temporary inflammation of the nerves have been reported, causing pain, weakness and paralysis in the limbs and which often spread to the chest and face (Guillain-Barré syndrome).
Reporting of side effects
If you or your child experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's online platform, www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Boostrix Polio
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton and on the label of the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze. Freezing destroys the vaccine.
Store in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container contents and additional information
Composition of Boostrix Polio
- The active ingredients are:
Diphtheria toxoid1 not less than 2 International Units (IU) (2.5 Lf)
Tetanus toxoid1 not less than 20 International Units (IU) (5 Lf)
Bordetella pertussisantigens
Pertussis toxoid1 8 micrograms
Filamentous hemagglutinin1 8 micrograms
Pertactin1 2.5 micrograms
Inactivated poliovirus
Type 1 (Mahoney strain)2 40 units of antigen D
Type 2 (MEF-1 strain)2 8 units of antigen D
Type 3 (Saukett strain)2 32 units of antigen D
1 adsorbed on hydrated aluminum hydroxide (Al(OH)3) 0.3 milligrams Al3+
and aluminum phosphate (AlPO4) 0.2 milligrams Al3+
2 propagated in Vero cells
Aluminum hydroxide and aluminum phosphate are included in the vaccine as adjuvants.
Adjuvants are substances included in certain vaccines to accelerate, enhance and/or prolong the protective effect of the vaccine.
- The other components are: Medium 199 (containing amino acids (including phenylalanine), mineral salts (including sodium and potassium), vitamins (including para-aminobenzoic acid) and other substances), sodium chloride and water for injectable preparations.
Appearance of the product and container contents
Injectable suspension in a pre-filled syringe.
Boostrix Polio is a white, slightly milky liquid presented in a pre-filled syringe (0.5 ml).
Boostrix Polio is available in a pre-filled syringe of 1 dose with or without separate needles; package sizes of 1 and 10.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
P.T.M - C/ Severo Ochoa, 2
28760 Tres Cantos
Madrid
Tel: 900 202 700
Manufacturer
GlaxoSmithKline Biologicals S.A.
Rue de l'Institut 89; 1330 Rixensart
Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Boostrix Polio:Belgium, Bulgaria, Czech Republic, Denmark, Germany, Greece, Spain, Iceland, Latvia, Lithuania, Luxembourg, Hungary, Netherlands, Norway, Austria, Poland, Portugal, Slovenia, Finland, Sweden
Boostrix Polio Lag:Slovak Republic
Boostrix Tetra:France
IPV-Boostrix:Ireland, Malta
Polio Boostrix:Italy
Boostrix-IPV:Romania
Date of the last revision of this leaflet:April 2023
Other sources of information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
-------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Before use, the vaccine should be left at room temperature and shaken well to obtain a white, turbid, and homogeneous suspension. Before administration, the vaccine should be visually examined to check for any foreign particles and/or changes in physical appearance. If any of these circumstances are observed, do not administer the vaccine.
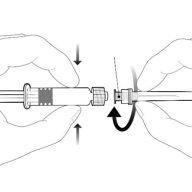
Instructions for the pre-filled syringe
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by turning it counterclockwise. |
| To insert the needle, attach the base to the luer-lockadapter and turn it a quarter turn clockwise until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste:
Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BOOSTRIX POLIO Injectable Suspension in Pre-filled SyringeDosage form: INJECTABLE, 0.5 mlActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 1 doseActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for BOOSTRIX POLIO Injectable Suspension in Pre-filled Syringe
Discuss questions about BOOSTRIX POLIO Injectable Suspension in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions