INFANRIX-IPV PRE-FILLED SYRINGE SUSPENSION FOR INJECTION

How to use INFANRIX-IPV PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Infanrix-IPV and what is it used for
- What you need to know before your child receives Infanrix-IPV
- How Infanrix-IPV is administered
- Possible side effects
- Storage of Infanrix-IPV
- Package contents and further information
- GlaxoSmithKline Biologicals S.A.,
- Rue de l' Institut 89
- 1330 Rixensart, Belgium
Introduction
Package Leaflet: Information for the User
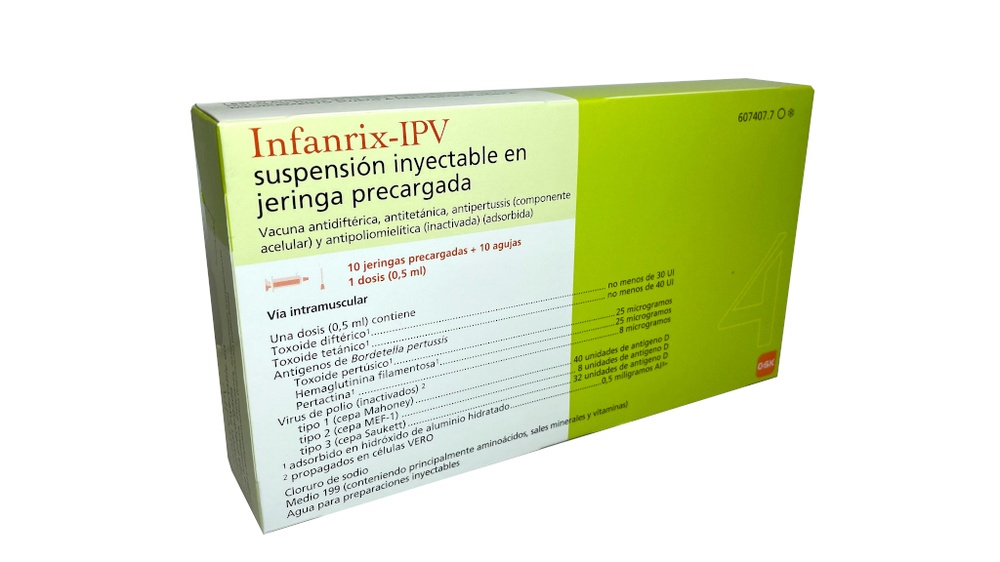
Infanrix-IPV, injectable suspension in a pre-filled syringe
Diphtheria, tetanus, pertussis (acellular component) and poliomyelitis (inactivated) (adsorbed) vaccine
Read all of this leaflet carefully before your child starts receiving this vaccine because it contains important information for you.
|
Contents of the package leaflet
- What is Infanrix-IPV and what is it used for
- What you need to know before your child receives Infanrix-IPV
- How Infanrix-IPV is administered
- Possible side effects
- Storage of Infanrix-IPV
- Package contents and further information
1. What is Infanrix-IPV and what is it used for
Infanrix-IPV is a vaccine used as a booster dose to protect your child against 4 diseases:
- Diphtheria- a serious bacterial infection that mainly affects the respiratory tract and, sometimes, the skin. The respiratory tract swells, causing severe breathing difficulties and, sometimes, asphyxia. The bacteria also release a toxin that can cause nerve damage, heart problems, and even death.
- Tetanus- the tetanus bacteria enter the body through cuts, scratches, or wounds in the skin. Wounds that are most likely to become infected with tetanus are burns, fractures, deep wounds, or wounds that contain dirt, dust, horse manure, or wood splinters. The bacteria release a toxin that can cause muscle stiffness, painful muscle spasms, convulsions, and even death. The muscle spasms can be strong enough to cause spinal fractures.
- Pertussis (whooping cough)- a highly contagious disease that affects the respiratory tract. It causes severe coughing that can lead to breathing problems. Often, the cough has a sound similar to a "barking" sound. The cough can last from one to two months or more. Pertussis can also cause ear infections, chest infections (bronchitis) that can last for a long time, lung infections (pneumonia), convulsions, brain damage, and even death.
- Polio- an infection caused by a virus. Often, polio is only a mild disease, although it can sometimes be very serious and cause permanent damage or even death. Polio can make the muscles unable to move (paralysis). This includes the muscles needed for breathing and walking. The affected arms or legs can become twisted (deformed) in a painful way.
Infanrix-IPV is for children from 16 months to 13 years (inclusive). It is not indicated for people over 14 years old.
How Infanrix-IPV works
- Infanrix-IPV helps the body develop its own protection (antibodies). This will protect your child against these diseases.
- The vaccine cannot cause the diseases it protects your child against.
2. What you need to know before your child receives Infanrix-IPV
Infanrix-IPV should not be administered
- If your child is allergic to the active substances or any of the other components of the vaccine (listed in section 6) or to neomycin or polymyxin (types of antibiotics) or formaldehyde. The signs of an allergic reaction may include skin rash with itching, difficulty breathing, and swelling of the face or tongue.
- If your child has had any allergic reaction to vaccines against diphtheria, tetanus, pertussis, or polio.
- If your child has had neurological problems (encephalopathy) in the 7 days following the administration of a pertussis vaccine.
- If your child has a severe infection with fever (over 38°C). A mild infection, such as a cold, should not be a problem, but consult your doctor first.
Infanrix-IPV should not be administered if any of the above situations apply to your child. If you are not sure, talk to your doctor or pharmacist before your child receives Infanrix-IPV.
Warnings and precautions
Consult your doctor or pharmacist before your child receives Infanrix-IPV if:
- after a previous administration of Infanrix-IPV or another pertussis vaccine, your child has had any problems, especially:
- fever (over 40°C) in the 48 hours following vaccination
- collapse or shock-like state in the 48 hours following vaccination
- inconsolable crying, persistent for 3 hours or more, produced in the 48 hours following vaccination
- convulsions, with or without fever, in the 3 days following vaccination
- your child has an undiagnosed or progressive brain disease or uncontrolled epilepsy. The vaccine should be administered once the disease is controlled
- your child has any bleeding problems or bruises easily
- your child has a tendency to have febrile seizures or has a family history of febrile seizures
- your child has problems with their immune system (including HIV infection). In this case, your child may be vaccinated with Infanrix-IPV. However, the protection obtained against infections may not be as high.
Before or after any injection, fainting (especially in adolescents) may occur, so you should inform your doctor or nurse if your child has fainted after receiving an injection in the past.
If any of the above situations apply to your child (or you are not sure), talk to your doctor or pharmacist before your child receives Infanrix-IPV.
Other medicines and Infanrix-IPV
Tell your doctor or pharmacist if your child is using, has recently used, or might use any other medicines.
In particular, tell your doctor or pharmacist if your child is using any of the following:
- medicines or other treatments (such as radiotherapy) that affect the immune system. Your child may be vaccinated with Infanrix-IPV. However, Infanrix-IPV may not work as well. If possible, the vaccine should be administered once this treatment has finished.
- Infanrix-IPV can be administered at the same time as other vaccines. Different injection sites will be used for each vaccine.
Pregnancy and breastfeeding
It is unlikely that Infanrix-IPV will be administered to pregnant or breastfeeding women. This is because the vaccine is only indicated for children from 16 months to 13 years (inclusive).
Administration of this vaccine during pregnancy or breastfeeding is not recommended.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines
It is unlikely that Infanrix-IPV will be administered to people who drive or use tools or machines. This is because the vaccine is only indicated for children from 16 months to 13 years (inclusive).
Your child may feel drowsy after vaccination. If this happens, your child should not drive, ride a bicycle, or use tools or machines.
Infanrix-IPV contains neomycin, polymyxin (antibiotics), para-aminobenzoic acid, phenylalanine, sodium, potassium, and formaldehyde.Infanrix-IPV should not be administered to your child if they are allergic to any of these components. Please consult your doctor if your child has had an allergic reaction to these components.
Infanrix-IPV contains para-aminobenzoic acid. It can cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm.
This vaccine contains 0.036 micrograms of phenylalanine per dose. Phenylalanine may be harmful in case of phenylketonuria (PKU), a rare genetic disease in which phenylalanine accumulates because the body is unable to eliminate it properly.
This vaccine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
This vaccine contains potassium, less than 1 mmol (39 mg) per dose; it is essentially "potassium-free".
3. How Infanrix-IPV is administered
When the vaccine is administered
- Your doctor or nurse will inform you when your child should receive this vaccine. This depends on official recommendations.
How Infanrix-IPV is administered
- Your child will receive a single injection of Infanrix-IPV.
- Infanrix-IPV is always injected into a muscle.
- The injection will normally be in the shoulder muscle. However, in small children, it can be administered in the thigh.
- The vaccine should never be administered into a vein.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. With this vaccine, the following side effects can occur:
Allergic reactions
If your child has an allergic reaction, go to a doctor immediately. The signs may include:
- skin rash with itching or blisters
- swelling of the eyes and face
- difficulty breathing or swallowing
- sudden drop in blood pressure
- loss of consciousness.
These signs usually start soon after receiving the injection. Take your child to the doctor immediately if they appear after leaving the clinic. Allergic reactions are very rare (less than 1 in 10,000 doses of vaccine).
Consult your doctor immediately if your child has any of the following serious side effects:
- collapse
- loss of consciousness
- convulsions
Consult your doctor immediately if you notice any of the above situations. These side effects have occurred with other pertussis vaccines. They usually appear within 2 or 3 days of vaccination.
Other side effects include:
Very common(may affect more than 1 in 10 doses of the vaccine): feeling drowsy, headache, loss of appetite, elevated temperature of 38°C or more, pain, redness, and swelling at the injection site, abnormal crying, feeling irritable or restless.
Common(may affect up to 1 in 10 doses of the vaccine): diarrhea, nausea, vomiting, elevated temperature of 39.5°C or more, feeling unwell, nodular rash at the injection site, feeling weak.
Uncommon(may affect up to 1 in 100 doses of the vaccine): skin allergies or rashes.
Rare(may affect up to 1 in 1,000 doses of the vaccine): swelling of the lymph nodes in the neck, armpits, and groin (lymphadenopathy), cough or chest infection (bronchitis), itching, nodular rash (urticaria).
Very rare(may affect up to 1 in 10,000 doses of the vaccine): bleeding or bruising more easily than normal (thrombocytopenia), temporary stop in breathing (apnea), inflammation of the face, lips, mouth, tongue, or throat, which can cause difficulty swallowing or breathing (angioedema), blisters at the injection site.
Booster doses of Infanrix-IPV may increase the risk of reactions at the injection site. Some of these reactions can affect the entire arm or leg where the injection was given. These reactions usually start 48 hours after the injection and disappear within 4 days.
Reporting of side effects
If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website (http://www.aemps.gob.es/). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Infanrix-IPV
- Keep this medicine out of the sight and reach of children.
- Store in a refrigerator (between 2°C and 8°C).
- Store in the original packaging to protect it from light.
- Do not freeze. Freezing destroys the vaccine.
- Do not use this medicine after the expiry date stated on the packaging. The expiry date is the last day of the month stated.
- Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in a pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package contents and further information
Composition of Infanrix-IPV
- The active substances are:
Diphtheria toxoid1 at least 30 IU
Tetanus toxoid1 at least 40 IU
Bordetella pertussisantigens
Pertussis toxoid1 25 micrograms
Filamentous hemagglutinin1 25 micrograms
Pertactin1 8 micrograms
Inactivated poliovirus2
Type 1 (Mahoney strain) 40 D-antigen units
Type 2 (MEF-1 strain) 8 D-antigen units
Type 3 (Saukett strain) 32 D-antigen units
1 adsorbed on hydrated aluminum hydroxide 0.5 milligrams Al3+
2 propagated in VERO cells
Aluminum hydroxide is included in this vaccine as an adjuvant. Adjuvants are substances present in some vaccines to accelerate, enhance, or prolong the protective effects of the vaccine.
- The other components are: sodium chloride, Medium 199 (containing amino acids (including phenylalanine), mineral salts (including sodium and potassium), and vitamins (including para-aminobenzoic acid) and other substances), water for injections.
Appearance of the product and package contents
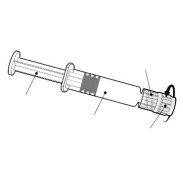
- Infanrix-IPV is an injectable suspension in a pre-filled syringe (0.5 ml).
- The suspension is white and slightly milky.
- Infanrix-IPV is available in a pre-filled syringe with or without a separate needle; package sizes of 1 and 10.
- Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Spain
Tel: +34 900 202 700
Manufacturer
GlaxoSmithKline Biologicals S.A.,
Rue de l' Institut 89
1330 Rixensart, Belgium
This medicine is authorized in the Member States of the European Economic Area under the following names:
Greece, France, Portugal, Cyprus: InfanrixTetra
Czech Republic, Estonia, Latvia, Lithuania, Norway, Slovakia, Sweden: Infanrix Polio
Finland: Infanrix-Polio
Poland, Spain: Infanrix-IPV
Hungary: Infanrix IPV
Ireland: IPV Infanrix
Italy: PolioInfanrix
Date of last revision of this leaflet:April 2023
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Once stored, a white deposit and a clear supernatant may be observed. This is not a sign of deterioration.
The syringe should be shaken well to obtain a white, turbid, and homogeneous suspension.
The suspension should be visually examined for any foreign particles and/or changes in physical appearance. If any of these circumstances are observed, discard the vaccine.
Instructions for the pre-filled syringe
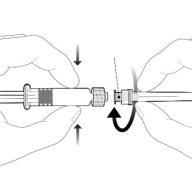
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by turning it counterclockwise. |
| To insert the needle, connect the base to the luer-lock adapter and turn it a quarter turn clockwise until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INFANRIX-IPV PRE-FILLED SYRINGE SUSPENSION FOR INJECTIONDosage form: INJECTABLE, 0.5 mlActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 1 doseActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for INFANRIX-IPV PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Discuss questions about INFANRIX-IPV PRE-FILLED SYRINGE SUSPENSION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions