XALKORI 150 MG GRANULADO EN CAPSULAS PARA ABRIR

Cómo usar XALKORI 150 MG GRANULADO EN CAPSULAS PARA ABRIR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
XALKORI 20mg granulado en cápsulas para abrir
XALKORI 50mg granulado en cápsulas para abrir
XALKORI 150mg granulado en cápsulas para abrir
crizotinib
Las palabras “usted” y “su” se utilizan para referirse tanto al paciente como al cuidador del paciente pediátrico.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es XALKORI y para qué se utiliza
- Qué necesita saber antes de empezar a tomar XALKORI
- Cómo administrar XALKORI granulado en cápsulas para abrir
- Posibles efectos adversos
- Conservación de XALKORI
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es XALKORI y para qué se utiliza
XALKORI es un medicamento contra el cáncer, que contiene crizotinib como principio activo, usado para tratar adultos con un tipo de cáncer de pulmón denominado cáncer de pulmón no microcítico, que tiene una determinada alteración o defecto en un gen denominado quinasa del linfoma anaplásico (ALK) o en un gen llamado ROS1.
XALKORI se utiliza para tratar a niños y adolescentes (de ≥ 1 a < 18 años de edad) con un tipo de tumor llamado linfoma anaplásico de células grandes (LACG) o un tipo de tumor llamado tumor miofibroblástico inflamatorio (TMI) que se presenta con un reordenamiento o defecto específico en un gen llamado quinasa del linfoma anaplásico (ALK).
XALKORI se puede recetar a niños y adolescentes para tratar el LACG si el tratamiento previo no ha ayudado a detener la enfermedad.
XALKORI se puede prescribir a niños y adolescentes para tratar el TMI si el tratamiento quirúrgico no ha ayudado a detener la enfermedad.
Solo debe recibir este medicamento bajo la supervisión de un médico con experiencia en el tratamiento del cáncer. Si tiene alguna duda sobre cómo actúa XALKORI o por qué le ha sido recetado, consulte a su médico.
2. Qué necesita saber antes de empezar a tomar XALKORI
No tome XALKORI
- Si es alérgico al crizotinib o a alguno de los demás componentes de este medicamento (incluidos en la sección 6, “Composición de XALKORI”).
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar XALKORI:
- Si tiene una enfermedad moderada o grave en el hígado.
- Si alguna vez ha tenido cualquier problema en el pulmón. Algunos problemas en el pulmón pueden empeorar durante el tratamiento con XALKORI, ya que XALKORI puede causar inflamación de los pulmones durante el tratamiento. Consulte a su médico de inmediato si tiene un síntoma nuevo o si empeora alguno de los síntomas incluyendo dificultad al respirar, falta de aliento, o tos con o sin mucosidad, o fiebre.
- Si después de realizarle un electrocardiograma (ECG), le han informado de que tiene una alteración en el corazón conocida como prolongación del intervalo QT.
- Si tiene disminución de la frecuencia cardiaca.
- Si ha tenido alguna vez problemas estomacales o intestinales, como orificios (perforación), o ha padecido enfermedades que provoquen inflamación en el interior del abdomen (diverticulitis) o si el cáncer se ha extendido al abdomen (metástasis).
- Si tiene alteraciones en la visión (ve flashes de luz, visión borrosa o visión doble).
- Si tiene una enfermedad grave en el riñón.
- Si está siendo tratado actualmente con cualquier otro medicamento incluido en la sección “Otros medicamentos y XALKORI”.
Si alguna de las situaciones anteriores es aplicable a usted, informe a su médico.
Hable con su médico de inmediato tras haber tomado XALKORI:
- Si experimenta intenso dolor de estómago o abdominal, fiebre, escalofríos, falta de aliento, pulso acelerado, pérdida de visión parcial o completa (en uno o ambos ojos) o cambios del hábito intestinal.
Niños y adolescentes
La indicación para el cáncer de pulmón no microcítico no incluye a niños ni adolescentes. No administre este medicamento a niños menores de 1 año de edad con LACG ALK‑positivo o TMI‑ALK positivo. XALKORI se debe administrar a niños y adolescentes bajo la supervisión de un adulto.
Otros medicamentos y XALKORI
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, incluyendo plantas medicinales y medicamentos adquiridos sin receta.
En particular, los siguientes medicamentos pueden aumentar el riesgo de efectos adversos con XALKORI:
- Claritromicina, telitromicina, eritromicina, antibióticos utilizados para tratar infecciones por bacterias.
- Ketoconazol, itraconazol, posaconazol, voriconazol, utilizados para tratar infecciones por hongos.
- Atazanavir, ritonavir, cobicistat, utilizados para tratar infecciones por VIH/SIDA.
Los siguientes medicamentos pueden disminuir la eficacia de XALKORI:
- Fenitoína, carbamazepina o fenobarbital, antiepilépticos utilizados para tratar convulsiones o ataques epilépticos.
- Rifabutina, rifampicina, utilizados para el tratamiento de la tuberculosis.
- Hierba de San Juan (Hypericum perforatum), una planta medicinal utilizada para tratar la depresión.
XALKORI puede aumentar los efectos adversos asociados a los siguientes medicamentos:
- Alfentanilo y otros opiáceos de corta duración como el fentanilo (analgésicos utilizados para procedimientos quirúrgicos).
- Quinidina, digoxina, disopiramida, amiodarona, sotalol, dofetilida, ibutilida, verapamilo, diltiazem, utilizados para tratar enfermedades del corazón.
- Medicamentos para la hipertensión denominados betabloqueantes, como atenolol, propanolol, labetalol.
- Pimozida, utilizada para tratar enfermedades mentales.
- Metformina, utilizada para tratar la diabetes.
- Procainamida, utilizada para tratar las arritmias cardiacas.
- Cisaprida, utilizada para tratar enfermedades gástricas.
- Ciclosporina, sirolimus y tacrolimus, utilizados en pacientes trasplantados.
- Alcaloides ergóticos (por ejemplo, ergotamina, dihidroergotamina) utilizados para tratar migrañas.
- Dabigatrán, anticoagulante utilizado para reducir la coagulación de la sangre.
- Colchicina, utilizada para tratar la gota.
- Pravastatina, utilizada para reducir los niveles de colesterol.
- Clonidina, guanfacina, utilizadas para tratar la hipertensión.
- Mefloquina, utilizada para la prevención de la malaria.
- Pilocarpina, utilizada para tratar el glaucoma (enfermedad grave del ojo).
- Anticolinesterasas, utilizadas para restaurar la función muscular.
- Antipsicóticos, utilizados para tratar enfermedades mentales.
- Moxifloxacino, utilizado para tratar infecciones por bacterias.
- Metadona, utilizada para tratar el dolor y para el tratamiento de la dependencia de opiáceos.
- Bupropión, utilizado para tratar la depresión y para dejar de fumar.
- Efavirenz, raltegravir, utilizados para tratar la infección por VIH.
- Irinotecán, un medicamento quimioterápico utilizado para el tratamiento del cáncer de colon y recto.
- Morfina, utilizada para tratar el dolor agudo y por cáncer.
- Naloxona, utilizado para el tratamiento de la adicción a opiáceos y su retirada.
Estos medicamentos se deben evitardurante el tratamiento con XALKORI.
Anticonceptivos orales
Si está tomando anticonceptivos orales mientras toma XALKORI, los anticonceptivos orales podrían ser ineficaces.
Toma de XALKORI con alimentos y bebidas
XALKORI puede tomarse después de una comida o en ayunas. No debe dispersar el granulado de XALKORI en alimentos. Debe evitar beber zumo de pomelo o comer pomelo mientras esté en tratamiento con XALKORI, ya que pueden alterar las cantidades de XALKORI en su cuerpo.
Protección solar
Evite pasar demasiado tiempo bajo la luz del sol. XALKORI puede hacer que su piel se vuelva sensible al sol (fotosensibilidad), y puede quemarse más fácilmente. Use ropa protectora y/o protector solar que cubra la piel para protegerse contra las quemaduras solares si tiene que estar expuesto a la luz del sol durante el tratamiento con XALKORI.
Embarazo y lactancia
Si está embarazada, puede quedarse embarazada o está en periodo de lactancia, consulte con su médico o farmacéutico antes de tomar este medicamento.
Se recomienda que las mujeres eviten quedarse embarazadas y que los hombres no sean padres durante el tratamiento con XALKORI, ya que este medicamento puede dañar al feto. Se deberá usar un método anticonceptivo adecuado durante el tratamiento y durante al menos 90 días después de haber completado el tratamiento, si hubiera alguna posibilidad de que la persona que está utilizando este medicamento se quede embarazada o conciba un hijo, ya que los anticonceptivos orales podrían ser ineficaces mientras toma XALKORI.
No dé el pecho durante el tratamiento con XALKORI. XALKORI podría dañar al bebé lactante.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Tenga especial cuidado cuando conduzca o utilice máquinas, ya que los pacientes en tratamiento con XALKORI pueden experimentar trastornos visuales, mareos y cansancio.
XALKORI contiene sacarosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo administrar XALKORI granulado en cápsulas para abrir
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- La dosis recomendada para niños y adolescentes con LACG ALK‑positivo o TMI ALK‑positivo es de 280 mg/m2 por vía oral dos veces al día. La dosis recomendada la calculará el médico del niño y dependerá del peso y la altura del niño (área de superficie corporal, ASC). La dosis máxima diaria en niños y adolescentes no debe exceder los 1 000 mg. XALKORI se debe administrar bajo la supervisión de un adulto.
- Administre la dosis recomendada una vez por la mañana y otra por la noche.
- Administre las cápsulas aproximadamente a las mismas horas cada día.
- El granulado se debe administrar en la boca y no se debe triturar, masticar ni dispersar sobre alimentos.
- No se debe tragar la cubierta de la cápsula.
- Forma de administración
Para obtener instrucciones detalladas sobre la administración del granulado de XALKORI, ver sección 7 “Instrucciones de uso” al final de este prospecto.
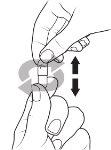
- Sostenga la cápsula de modo que el texto “Pfizer” quede en la parte superior, y dé unos golpecitos a la cápsula para que todo el granulado caiga a la mitad inferior de la cápsula.
- Con cuidado, apriete la parte inferior de la cápsula.
- Gire la tapa de la cápsula y retírela.
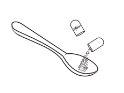
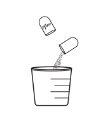
- Vierta el granulado directamente en la boca del niño, O BIEN vierta primero el granulado en una cuchara o un vasito medidor y, a continuación, viértalo en la boca del niño.
- Dé golpecitos a la cápsula abierta para asegurarse de que se ha administrado todo el granulado.
- Si no se puede administrar la dosis completa de una sola vez, divídala en porciones hasta que haya administrado la dosis completa.
- Inmediatamente después de la administración, dé un vaso de agua al niño para asegurarse de que ha tragado todo el granulado.
- Una vez que el niño haya tragado el granulado, puede darle otros líquidos o alimentos, excepto pomelo y zumo de pomelo.
Si es preciso, su médico puede reducir la dosis a tomar por vía oral. Su médico puede decidir suspender de forma permanente el tratamiento con XALKORI si no puede tolerar XALKORI.
Si toma más XALKORI del que debe
Si de forma accidental toma más cápsulas, consulte de inmediato con su médico o farmacéutico. Puede requerir atención médica.
Si olvidó tomar XALKORI
El modo de proceder si olvidó tomar una cápsula depende de cuánto tiempo falte hasta la siguiente dosis:
- Si la siguiente dosis es dentro de 6horas o más, tome la cápsula olvidada lo antes posible. Después tome la siguiente cápsula a la hora habitual.
- Si la siguiente dosis es en menos de 6horas, no tome la cápsula olvidada. Después tome la siguiente cápsula a la hora habitual.
Informe a su médico de la dosis olvidada en su siguiente visita.
No tome una dosis doble para compensar la cápsula olvidada.
Si vomita tras tomar una dosis de XALKORI, no tome una dosis adicional; tome la dosis siguiente a la hora habitual.
Si interrumpe el tratamiento con XALKORI
Es importante que tome XALKORI todos los días, durante el tiempo que su médico se lo haya recetado. Si no es capaz de tomar este medicamento tal y como su médico se lo ha recetado, o cree que ya no lo necesita más, contacte inmediatamente con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta efectos adversos, consulte con su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Aunque no todos los efectos adversos identificados en los adultos con CPNM se han observado en los niños y adolescentes con LACG o TMI, se deben considerar los mismos efectos adversos para los pacientes adultos con cáncer de pulmón y para los niños y adolescentes con LACG o TMI.
Algunos efectos adversos pueden ser graves. Usted deberá contactar inmediatamente con su médico si experimenta alguno de los siguientes efectos adversos graves (ver también sección 2 “Qué necesita saber antes de empezar a tomar XALKORI”):
- Insuficiencia hepática
Consulte con su médico inmediatamente si se siente más cansado de lo habitual, si su piel y las zonas blancas de sus ojos se vuelven amarillas, si su orina se vuelve oscura o marrón (color té), si tiene náuseas, vómitos, o menos apetito, si le duele la parte derecha del estómago, o si tiene picores o si le salen moratones con más facilidad de lo normal. Su médico podrá hacerle análisis de sangre para comprobar su función hepática, y si los resultados de estos análisis fueran anormales, podría reducir la dosis de XALKORI o suspender el tratamiento.
- Inflamación del pulmón
Consulte con su médico inmediatamente si experimenta dificultad para respirar, especialmente si está asociada con tos o fiebre.
- Reducción en el número de células blancas de la sangre (incluyendo neutrófilos)
Consulte con su médico inmediatamente si experimenta fiebre o infección. Su médico puede hacerle análisis de sangre y si los resultados fueran anómalos, su médico puede tomar la decisión de reducir la dosis de XALKORI.
- Sensación de mareo, desmayo o dolor en el pecho
Consulte con su médico inmediatamente si padece alguno de estos síntomas ya que podrían ser signos de cambios en la actividad eléctrica (se observan en un electrocardiograma) o de un ritmo anormal del corazón. Su médico podría hacerle electrocardiogramas para comprobar que no hay problemas en su corazón durante el tratamiento con XALKORI.
- Pérdida de la visión parcial o completa en uno o ambos ojos
Consulte a su médico inmediatamente si experimenta nuevos problemas visuales, pérdida de visión o algún cambio en la visión, como dificultad para ver con uno o ambos ojos. Su médico puede suspender o interrumpir permanentemente el tratamiento con XALKORI y derivarlo a un oftalmólogo.
Para niños y adolescentes que reciben XALKORI para el tratamiento del LACG ALK‑positivo o TMI ALKpositivo: su médico debe derivarlo a un oftalmólogo antes de comenzar el tratamiento con XALKORI, y en un plazo de 1 mes tras comenzar el tratamiento con XALKORI para detectar problemas visuales. Debe hacerse una exploración oftalmológica cada 3 meses durante el tratamiento con XALKORI y más a menudo si hay nuevos problemas visuales.
- Problemas gástricos e intestinales (gastrointestinales) graves en niños y adolescentes con LACG ALK‑positivo o TMI ALK‑positivo
XALKORI puede provocar diarrea grave, náuseas o vómitos. Informe a su médico inmediatamente si presenta problemas para tragar, vómitos o diarrea durante el tratamiento con XALKORI. Su médico puede darle medicamentos según sea necesario para prevenir o tratar la diarrea, las náuseas y los vómitos. Su médico puede recomendarle beber más líquidos o recetarle suplementos de electrolitos u otros tipos de apoyo nutricional si se presentan síntomas graves.
Otros efectos adversos de XALKORI observados en adultos con CPNM pueden incluir:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Efectos visuales (ver flashes de luz, visión borrosa, sensibilidad a la luz, visión de motas o visión doble, normalmente aparecen pronto después de comenzar el tratamiento con XALKORI).
- Problemas gástricos, incluyendo vómitos, diarrea, náuseas.
- Edema (exceso de líquido en el tejido corporal que causa inflamación de las manos y de los pies).
- Estreñimiento.
- Anomalías en las pruebas del hígado en los análisis de sangre.
- Disminución del apetito.
- Cansancio.
- Mareo.
- Neuropatías (sensación de entumecimiento u hormigueo en las articulaciones o extremidades).
- Alteración del sentido del gusto.
- Dolor en el abdomen.
- Reducción en el número de glóbulos rojos de la sangre (anemia).
- Erupción cutánea.
- Reducción del ritmo cardiaco.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Indigestión.
- Aumento de los niveles de creatinina en sangre (puede indicar que los riñones no funcionan adecuadamente).
- Aumento de los niveles de la enzima fosfatasa alcalina en sangre (indicador de una disfunción o lesión de un órgano, especialmente del hígado, páncreas, huesos, glándula tiroides o vesícula biliar).
- Hipofosfatemia (niveles bajos de fosfato en sangre que pueden provocar confusión o debilidad muscular).
- Líquido encapsulado dentro del riñón (quistes renales).
- Desmayo.
- Inflamación del esófago (conducto de la deglución).
- Disminución de los niveles de testosterona, una hormona sexual masculina.
- Fallo cardiaco.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Orificio (perforación) en el estómago o el intestino.
- Sensibilidad a la luz solar (fotosensibilidad).
- Resultados elevados en los análisis de sangre para comprobar si hay daño muscular (niveles altos de creatinfosfoquinasa).
Otros efectos adversos de XALKORI observados en niños y adolescentes con LACG ALK‑positivo o TMI ALK‑positivo pueden incluir:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Anomalías en las pruebas hepáticas en los análisis de sangre.
- Efectos visuales (ver flashes de luz, visión borrosa, sensibilidad a la luz, visión de motas o visión doble, normalmente aparecen pronto después de comenzar el tratamiento con XALKORI).
- Dolor en el abdomen.
- Aumento de los niveles de creatinina en sangre (puede indicar que los riñones no funcionan adecuadamente).
- Anemia (reducción en el número de glóbulos rojos de la sangre).
- Recuento bajo de plaquetas en los análisis de sangre (puede aumentar el riesgo de hemorragia y hematomas).
- Cansancio.
- Disminución del apetito.
- Estreñimiento.
- Edema (exceso de líquido en el tejido corporal que causa inflamación de las manos y de los pies).
- Aumento de los niveles de la enzima fosfatasa alcalina en sangre (indicador de una disfunción o lesión de un órgano, especialmente del hígado, páncreas, huesos, glándula tiroides o vesícula biliar).
- Neuropatía (sensación de entumecimiento u hormigueo en las articulaciones o extremidades).
- Mareo.
- Indigestión.
- Alteración del sentido del gusto.
- Hipofosfatemia (niveles bajos de fosfato en sangre que pueden provocar confusión o debilidad muscular).
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Erupción cutánea.
- Inflamación del esófago (conducto de la deglución).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de XALKORI
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y en la caja después de “EXP”. La fecha de caducidad es el último día del mes que se indica.
- Este medicamento no requiere condiciones especiales de conservación.
- No utilice este medicamento si está dañado o tiene signos de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Las cubiertas de las cápsulas vacías de XALKORI (granulado por vía oral) pueden tirarse a la basura doméstica. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de XALKORI
- El principio activo de XALKORI es crizotinib.
XALKORI 20 mg granulado en cápsulas para abrir: cada cápsula contiene 20 mg de crizotinib
XALKORI 50 mg granulado en cápsulas para abrir: cada cápsula contiene 50 mg de crizotinib
XALKORI 150 mg granulado en cápsulas para abrir: cada cápsula contiene 150 mg de crizotinib
- Los demás componentes son (ver también la sección 2 “XALKORI contiene sacarosa”):
Contenido del granulado: alcohol estearílico, poloxámero, sacarosa, talco (E553b), hipromelosa (E464), macrogol (E1521), monoestearato de glicerol (E471), triglicéridos de cadena media.
Cápsula: gelatina, dióxido de titanio (E171), azul brillante (E133) u óxido de hierro negro (E172).
Tinta de impresión: shellac (E904), propilenglicol (E1520), hidróxido de potasio (E525), óxido de hierro negro (E172).
Aspecto del producto y contenido del envase
El granulado de XALKORI es de color blanco a blanquecino y se encuentran en una cápsula para abrir.
XALKORI 20 mg granulado en cápsulas para abrir son cápsulas con la tapa azul claro con el texto “Pfizer” impreso en tinta negra y el cuerpo blanco con el texto “CRZ 20” impreso en tinta negra.
XALKORI 50 mg granulado en cápsulas para abrir son cápsulas con la tapa gris con el texto “Pfizer” impreso en tinta negra y el cuerpo gris claro con el texto “CRZ 50” impreso en tinta negra.
XALKORI 150 mg granulado en cápsulas para abrir son cápsulas con la tapa azul claro con el texto “Pfizer” impreso en tinta negra y el cuerpo azul claro con el texto “CRZ 150” impreso en tinta negra.
Está disponible en frascos de plástico con 60 cápsulas para abrir.
Titular de la autorización de comercialización
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Bélgica
Responsable de la fabricación
Pfizer Service Company BV
Hoge Wei 10
Zaventem
Vlaams-Brabant 1930
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
España
Pfizer, S.L.
Tel: +34 91 490 99 00
Fecha de la última revisión de este prospecto:08/2024.
Otras fuentes de información
La información detallada de este medicamento y la información en distintos idioma está disponible escaneando el código QR que se incluye más abajo y en la caja con un dispositivo móvil.
Código QR por incluir
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
- Instrucciones de uso
Lea la sección 7 entera antes de usar XALKORI granulado en cápsulas para abrir.
Artículos necesarios para la administración de XALKORI:
- XALKORI granulado en cápsulas, bajo prescripción de su médico
- De manera opcional, una cuchara o un vasito medidor suministrados por el usuario
Preparación del granulado de XALKORI (pasos1 a 3):
Paso1 | Saque el número de cápsulas necesarias para la dosis prescrita de granulado de XALKORI del frasco o frascos correspondientes. |
Paso2 |
|
Paso3 | Sujete las partes superior e inferior de la cápsula y gírelas en direcciones opuestas para separarlas y abrir la cápsula.
|
Administración del granulado de XALKORI (paso4):Existen 2opcionespara administrar el granulado por vía oral al niño.
Paso4 | Opción1 (verter el granulado directamente en la boca del niño) |
|
Opción2 (verter el granulado desde un utensilio para la administración) |
|
Una vez completado el paso 4, puede darle al niño otros líquidos o alimentos, excepto pomelo y zumo de pomelo.
Consulte a su médico o farmacéutico si no está seguro de cómo preparar o administrar al niño la dosis prescrita de granulado de XALKORI.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a XALKORI 150 MG GRANULADO EN CAPSULAS PARA ABRIRForma farmacéutica: CAPSULA, 20 mgPrincipio activo: CrizotinibFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: CÁPSULA, 200mgPrincipio activo: CrizotinibFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: CAPSULA, 250mgPrincipio activo: CrizotinibFabricante: Pfizer Europe Ma EeigRequiere receta
Médicos online para XALKORI 150 MG GRANULADO EN CAPSULAS PARA ABRIR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de XALKORI 150 MG GRANULADO EN CAPSULAS PARA ABRIR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





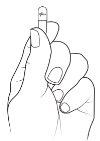 Dé unos golpecitos a la cápsula para que todo el granulado caiga a la parte inferior de la cápsula. Apriete con cuidado la parte inferior de la cápsula para que la parte de arriba quede suelta.
Dé unos golpecitos a la cápsula para que todo el granulado caiga a la parte inferior de la cápsula. Apriete con cuidado la parte inferior de la cápsula para que la parte de arriba quede suelta.