
VANTOBRA 170 MG SOLUCION PARA INHALACION POR NEBULIZADOR


Cómo usar VANTOBRA 170 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el paciente
Vantobra 170 mg solución para inhalación por nebulizador
tobramicina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Vantobra y para qué se utiliza
- Qué necesita saber antes de empezar a usar Vantobra
- Cómo usar Vantobra
- Posibles efectos adversos
- Conservación de Vantobra
- Contenido del envase e información adicional
1. Qué es Vantobra y para qué se utiliza
Qué es Vantobra
Vantobra contiene un antibiótico denominado tobramicina. Pertenece a una clase de antibióticos conocidos como aminoglucósidos.
Para qué se utiliza Vantobra
Vantobra se usa en pacientes con fibrosis quística a partir de 6 años de edad para el tratamiento de infecciones pulmonares causadas por una bacteria llamada Pseudomomas aeruginosa.
Pseudomomas aeruginosaes una bacteria que de forma frecuente infecta los pulmones de los pacientes con fibrosis quística en algún momento de su vida. Si no se trata de manera adecuada la infección, esta sigue dañando los pulmones y causa más problemas de tipo respiratorio.
Cómo actúa Vantobra
Cuando se inhala Vantobra, el antibiótico puede entrar directamente en los pulmones para luchar contra las bacterias causantes de la infección. Este medicamento actúa alterando la producción de las proteínas que necesitan las bacterias para construir sus paredes celulares. Esto daña las bacterias y termina matándolas.
2. Qué necesita saber antes de empezar a usar Vantobra
No use Vantobra:
- si es alérgico (hipersensible) a tobramicina, o a algún antibiótico aminoglucósido o a alguno de los demás componentes de Vantobra (incluidos en la sección 6).
Si se encuentra en alguna de las circunstancias anteriores, informe a su médico antes de usar Vantobra.
Advertencias y precauciones
Consulte a su médico si alguna vez ha sufrido cualquiera de las siguientes enfermedades:
- problemas auditivos (incluidos ruidos en los oídos y mareo)
- problemas renales
- molestias en el pecho
- sangre en el esputo (la sustancia que se expulsa al toser)
- debilidad muscular persistente o que empeore con el paso del tiempo, un síntoma frecuentemente relacionado con trastornos como la miastenia (debilidad muscular) o la enfermedad de Parkinson.
Si se encuentra en alguna de las circunstancias anteriores, informe a su médico antes de usar Vantobra.
Si tiene problemas auditivos o con la función renal, su médico podrá tomarle muestras de sangre para supervisar la cantidad de Vantobra presente en su organismo.
Si usted o los miembros de su familia materna tienen una enfermedad ocasionada por una mutación mitocondrial (una enfermedad genética) o pérdida de audición debido a antibióticos, se le recomienda informar a su médico o farmacéutico antes de tomar este medicamento. Ciertas mutaciones mitocondriales pueden aumentar su riesgo de pérdida auditiva con este producto. Su médico puede recomendar pruebas genéticas antes de la administración de Vantobra.
La inhalación de medicamentos puede causar molestias en el pecho debido al estrechamiento de las vías respiratorias; esto puede suceder con Vantobra. Es posible que su médico le pida que use otros medicamentos adecuados para ensanchar las vías respiratorias antes de usar Vantobra.
Las cepas de Pseudomonaspueden hacerse resistentes al tratamiento con antibióticos con el paso del tiempo. Esto quiere decir que, con el tiempo, Vantobra podría dejar de funcionar como debe. Si tiene dudas acerca de esto, consulte a su médico.
Si además está tomando tobramicina u otro antibiótico aminoglucósido administrado mediante inyección, esto puede aumentar el riesgo de sufrir efectos adversos. Su médico le controlará según proceda.
Niños
Este medicamento no está indicado para su uso en niños menores de 6 años de edad.
Otros medicamentos y Vantobra
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
No debe tomar los siguientes medicamentos mientras esté usando Vantobra:
- furosemida, un diurético (medicamento que aumenta el volumen de orina)
- otros medicamentos con potencial diurético, como urea o manitol
- otros medicamentos que puedan dañar a sus riñones o a la audición:
- amfotericina B, cefalotina, polimixinas (empleadas para tratar las infecciones microbianas), ciclosporina, tacrolimus (empleado para reducir la actividad del sistema inmunitario). Estos medicamentos pueden dañar los riñones;
- compuestos a base de platino, como el carboplatino y el cisplatino (empleado para tratar ciertas formas de cáncer). Estos medicamentos pueden dañar los riñones o la audición.
Los siguientes medicamentos pueden aumentar el riesgo de efectos perjudiciales si se administran cuando ya esté recibiendo tobramicina u otro antibiótico aminoglucósido administrado mediante inyección:
- anticolinesterasas, como neostigmina y pirodistigmina (empleadas para tratar la debilidad muscular) o la toxina botulínica. Estos medicamentos pueden hacer que aparezca o empeore una debilidad muscular.
Si toma alguno de los medicamentos anteriores, consulte a su médico antes de usar Vantobra.
No debe mezclar ni diluir Vantobra con ningún otro medicamento en el nebulizador de mano Tolero que se suministra junto con Vantobra.
Si está tomando distintos medicamentos para la fibrosis quística, los tomará en el siguiente orden:
- Tratamiento broncodilatador, como salbutamol
- Fisioterapia respiratoria
- Otros medicamentos inhalados
- Vantobra
Consulte con su médico el orden.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Se desconoce si la inhalación de este medicamento durante el embarazo puede causar efectos adversos. Cuando se administran mediante inyección, tobramicina y otros antibióticos aminoglucósidos pueden causar daños al feto, como sordera y problemas renales.
Si está en periodo de lactancia, debe consultar a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se espera que Vantobra afecte a su capacidad para conducir o usar máquinas.
3. Cómo usar Vantobra
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
La dosis recomendada es de dos ampollas al día (una por la mañana y otra por la noche) durante 28 días.
- La dosis es la misma para todas las personas a partir de 6 años de edad.
- Inhalar por la boca el contenido completo de una ampolla por la mañana y otra ampolla por la noche utilizando el nebulizador de mano Tolero.
- Se recomienda que el intervalo entre dosis sea lo más próximo posible a 12 horas, en cualquier caso, este intervalo debe ser como mínimo de 6 horas.
- Después de que haya usado el medicamento durante 28 días, iniciará un periodo de descanso de otros 28 días, durante los cuales no inhalará ninguna dosis de Vantobra. A continuación, tendrá que iniciar un nuevo ciclo tras el periodo de descanso (tal y como se ilustra).
- Es importante que siga usando el medicamento dos veces al día durante los 28 días de tratamiento y que respete el ciclo de 28 días con tratamiento y 28 días sin tratamiento.

Repita el ciclo
Continue usando Vantobra siguiendo este mismo esquema hasta que su médico le diga lo contrario.
Si tiene alguna duda respecto a cuánto tiempo tiene que seguir usando Vantobra, consulte a su médico o farmacéutico.
Preparación de Vantobra para la inhalación
- Utilice Vantobra solo con el nebulizador de mano Tolero que se muestra en la siguiente imagen para asegurarse de inhalar la dosis correcta. No utilice el nebulizador de mano Tolero con ningún otro medicamento.
- Lea las instrucciones de uso incluidas con el nebulizador de mano antes de usarlo.
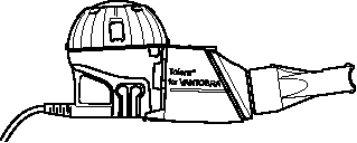
- Asegúrese de disponer de un controlador eTrack o eBase al que conectar el nebulizador de mano Tolero. El controlador correspondiente puede ser recetado por su médico o adquirirse por separado.
- Lavar bien las manos con agua y jabón.
- Retire una ampolla de Vantobra del sobre de papel de aluminio justo antes de la inhalación.
- Conserve el resto del medicamento refrigerado en la caja original.
- Coloque todas las piezas del nebulizador de mano Tolero en un papel o un paño limpios y secos. Asegúrese de que el nebulizador de mano se encuentre sobre una superficie plana y estable.
- Montar el nebulizador de mano Tolero tal y como se indica en sus instrucciones de uso.
- Sujetar la ampolla en posición recta y golpear ligeramente antes de retirar la parte superior para evitar que se derrame. Vaciar el contenido de una ampolla en el depósito para medicación del nebulizador de mano.
- Comience el tratamiento sentándose erguido en una habitación bien ventilada. Sujete el nebulizador en posición horizontal y respire por la boca con normalidad. Evite respirar por la nariz. Continúe inspirando y espirando cómodamente hasta terminar el tratamiento. Cuando se haya suministrado todo el medicamento, oirá una señal acústica para indicar que ha finalizado la administración.
- Si por cualquier motivo tiene que interrumpir el tratamiento antes de terminarlo, mantenga pulsado el botón On/Off durante un segundo. Para reiniciar el tratamiento, vuelva a mantener pulsado el botón On/Off durante un segundo.
- El nebulizador de mano Tolero se debe limpiar y desinfectar tal y como se describe en las instrucciones de uso.
- Usar un nebulizador de mano Tolero nuevo en cada ciclo de tratamiento (28 días de tratamiento activo), incluido con el medicamento.
No utilice un sistema nebulizador alternativo y no probado, ya que esto puede alterar la cantidad de medicamento que llega a los pulmones, lo que a su vez puede alterar la seguridad y la eficacia del medicamento.
Si usa más Vantobra del que debe
Si inhala demasiado Vantobra puede que su voz se vuelva muy ronca. Informe a su médico lo antes posible. Si traga Vantobra, es poco probable que cause problemas graves, dado que la tobramicina apenas se absorbe en el estómago, pero aun así, debe informar a su médico lo antes posible.
Si olvidó usar Vantobra
En caso de que olvide usar Vantobra y queden al menos 6 horas hasta la próxima dosis, tome la dosis tan pronto como pueda. De lo contrario, espere hasta la siguiente dosis. No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Vantobra
No interrumpa el tratamiento con Vantobra a no ser que se lo diga su médico, ya que la infección pulmonar puede no estar suficientemente controlada y podría empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves
- molestias en el pecho con dificultad para respirar (raro, puede afectar hasta 1 de cada 1.000 personas)
- reacciones alérgicas, como urticaria y prurito (muy raras, puede afectar hasta 1 de cada 10.000 personas)
Si experimenta alguno de estos efectos, deje de usar Vantobra y póngase inmediatamente en contacto con su médico.
Las personas con fibrosis quística presentan diversos síntomas de esta enfermedad. Dichos síntomas se pueden seguir presentando mientras se usa Vantobra, pero no deben ser tan frecuentes ni peores que antes.
Si su enfermedad pulmonar de base parece que empeora mientras toma Vantobra, informe inmediatamente a su médico.
Otros efectos adversos pueden ser:
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- dificultad para respirar
- alteración de la voz (voz ronca)
- mayor frecuencia de tos
- dolor de garganta
Raras (pueden afectar hasta 1 de cada 1.000 personas)
- Laringitis (inflamación de las cuerdas vocales que puede causar una alteración de la voz, dolor de garganta y dificultad para tragar)
- Pérdida de voz
- Dolor de cabeza, debilidad
- Hemorragia nasal, secreción nasal
- Pitidos en los oídos (normalmente transitorios), pérdida de audición, mareo
- Toser sangre, producir más esputo de lo normal, molestias en el pecho, asma, fiebre
- Alteraciones del gusto, sensación de malestar (náuseas), úlceras de la boca, malestar (vómitos), apetito disminuido
- Erupción
- Dolor en el pecho o dolor generalizado
- Empeoramiento de los resultados de la función pulmonar
Muy raras (pueden afectar hasta 1 de cada 10.000 personas)
- Infecciones por hongos en la boca o la garganta, como aftas
- Inflamación de los ganglios linfáticos
- Somnolencia
- Dolor de oídos, problemas en los oídos
- Hiperventilación, niveles bajos de oxígeno en la sangre, sinusitis
- Diarrea, dolor en o alrededor del estómago
- Pústulas de color rojo, pápulas en la piel
- Urticaria, prurito
- Dolor de espalda
- Malestar general
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vantobra
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la ampolla, en el sobre o en la caja después de «CAD». La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). Si no dispone de una nevera (por ejemplo, al transportar el medicamento), puede conservar la caja con el medicamento (incluso si los sobres están abiertos) por debajo de 25 °C durante un máximo de 4 semanas. Si el medicamento se conserva a temperatura ambiente durante más de 4 semanas, se desechará de acuerdo con la normativa local.
No utilice este medicamento si observa un aspecto turbio o se observan partículas en la solución.
No conserve nunca una ampolla abierta.Una vez abierta una ampolla debe usarse inmediatamente y, el producto restante se desechará.
Los medicamentos no se deben tirar a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Vantobra
- El principio activo es tobramicina. Una ampolla contiene 170 mg de tobramicina como dosis única.
- Los demás componentes (excipientes) son: cloruro de sodio, cloruro cálcico, sulfato de magnesio, agua para preparaciones inyectables, ácido sulfúrico e hidróxido de sodio para ajustar el pH.
Aspecto de Vantobra y contenido del envase
Vantobra solución para inhalación por nebulizador se suministra en una ampolla lista para usar.
Vantobra es una solución de color claro a amarillento, que puede variar hasta el amarillo oscuro. Esto no afecta a la acción de Vantobra siempre y cuando se hayan seguido las instrucciones de conservación.
Las ampollas se envasan en sobres, y cada sobre contiene 8 ampollas, lo que corresponde a cuatro días de tratamiento.
Vantobra está disponible junto con un nebulizador de mano Tolero. Se presenta en una caja que a su vez contiene dos cajas en su interior, una con el medicamento (56 ampollas con solución para inhalación por nebulizador en 7 sobres) y otra que contiene el nebulizador de mano. Un envase es suficiente para un ciclo de tratamiento de 28 días.
Titular de la autorización de comercialización y responsable de la fabricación
PARI Pharma GmbH
Moosstrasse 3
D-82319 Starnberg
Alemania
Tel.: +49 (0) 89 – 74 28 46 - 10
Fax: +49 (0) 89 – 74 28 46 30
E-mail: [email protected]
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VANTOBRA 170 MG SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INHALACIÓN PULMONAR, 300 mg / 4 mlPrincipio activo: TobramicinaFabricante: Chiesi España S.A.U.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 28 mg tobramicinaPrincipio activo: TobramicinaFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 300 mg/5 mlPrincipio activo: TobramicinaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para VANTOBRA 170 MG SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VANTOBRA 170 MG SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















