Ultibro Breezhaler 85mcg/43mcg polvo para inhalacion (capsula dura)


Cómo usar Ultibro Breezhaler 85mcg/43mcg polvo para inhalacion (capsula dura)
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ultibro Breezhaler 85microgramos/43microgramospolvo para inhalación (cápsula dura)
indacaterol/glicopirronio
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ultibro Breezhaler y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ultibro Breezhaler
- Cómo usar Ultibro Breezhaler
- Posibles efectos adversos
- Conservación de Ultibro Breezhaler
- Contenido del envase e información adicional
Instrucciones de uso del inhalador de Ultibro Breezhaler
1. Qué es Ultibro Breezhaler y para qué se utiliza
Qué esUltibro Breezhaler
Este medicamento contiene dos principios activos denominados indacaterol y glicopirronio, los cuales pertenecen a una clase de medicamentos denominados broncodilatadores.
Para qué se utiliza Ultibro Breezhaler
Este medicamento se utiliza para facilitar la respiración de los pacientes adultos que tienen dificultades para respirar debido a una enfermedad pulmonar denominada enfermedad pulmonar obstructiva crónica (EPOC). En la EPOC, los músculos que rodean a las vías respiratorias se contraen, lo cual dificulta la respiración. Este medicamento bloquea la contracción de dichos músculos en los pulmones, facilitando la entrada y salida de aire de los mismos.
Si usa este medicamento una vez al día, le ayudará a reducir los efectos de la EPOC en su vida diaria.
2. Qué necesita saber antes de empezar a usar Ultibro Breezhaler
No use Ultibro Breezhaler
- si es alérgico a indacaterol o glicopirronio o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico,farmacéutico o enfermero antes de empezar a usar Ultibro Breezhaler si cualquiera de las situaciones siguientes le afecta a usted:
- tiene asma – este medicamento no se debe utilizar como tratamiento para el asma.
- tiene problemas de corazón.
- tiene convulsiones o ataques.
- tiene problemas de la glándula del tiroides (tirotoxicosis).
- tiene diabetes.
- está usando algún medicamento para su enfermedad de pulmón que contiene principios activos similares (misma clase) a los de Ultibro Breezhaler (ver sección «Uso de Ultibro Breezhaler con otros medicamentos»).
- padece problemas de riñón.
- padece problemas de hígado graves.
- padece un trastorno ocular conocido como glaucoma de ángulo estrecho.
- tiene dificultad para orinar.
Si cualquiera de las situaciones anteriores le afecta a usted (o no está seguro), consulte con su médico, farmacéutico o enfermero antes de empezar a usar este medicamento.
Durante el tratamiento conUltibro Breezhaler
- Interrumpa el uso de este medicamento y solicite ayuda médica inmediatamentesi experimenta cualquiera de las siguientes situaciones:
- dolor o molestia en los ojos, visión borrosa pasajera, halos visuales o imágenes coloreadas en asociación con un enrojecimiento de los ojos – estos pueden ser signos de un ataque agudo de glaucoma de ángulo estrecho.
- dificultad para respirar o tragar, hinchazón de la lengua, labios o cara, erupción cutánea, picazón y urticaria (signos de una reacción alérgica).
- opresión en el pecho, tos, sibilancias o dificultad para respirar inmediatamente después de usar este medicamento – estos pueden ser signos de un estado denominado broncoespasmo paradójico.
- Informe a su médico inmediatamentesi sus síntomas de la EPOC, como son, dificultad para respirar, respiración jadeante o tos, no mejoran o empeoran.
Ultibro Breezhaler se utiliza como tratamiento continuo para su EPOC. No utilice este medicamento para tratar un ataque repentino de disnea o sibilancias.
Niños y adolescentes
No administre este medicamento a niños o adolescentes menores de 18 años de edad. Esto se debe a que su empleo no se ha estudiado en este grupo de edad.
Otros medicamentos y Ultibro Breezhaler
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Especialmente, informe a su médico o farmacéutico si está usando:
- cualquier medicamento que pueda ser similar a Ultibro Breezhaler (que contengan sustancias activas similares).
- medicamentos denominados betabloqueantes que se pueden utilizar para la presión sanguínea elevada u otros problemas del corazón (como es propranolol), o para un problema de los ojos denominado glaucoma (como es timolol).
- medicamentos que disminuyen la cantidad de potasio en la sangre. Estos incluyen:
- corticoides (p.ej. prednisolona),
- diuréticos utilizados para la presión sanguínea elevada (p.ej. hidroclorotiazida),
- medicamentos para problemas respiratorios (p.ej. teofilina).
Embarazo y lactancia
No se dispone de datos sobre el uso de este medicamento en mujeres embarazadas y se desconoce si el principio activo de este medicamento pasa a la leche materna. Indacaterol, una de las sustancias activas de Ultibro Breezhaler, puede inhibir el parto debido a su efecto en el útero.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No debe utilizar Ultibro Breezhaler a no ser que su médico se lo indique.
Conducción y uso de máquinas
No es probable que este medicamento afecte a su capacidad para conducir y utilizar máquinas. Sin embargo, este medicamento puede causar mareo (ver sección 4). Si se siente mareado mientras esté tomando este medicamento, no conduzca o use máquinas.
Ultibro Breezhalercontiene lactosa
Este medicamento contiene lactosa (23,5 mg por cápsula). Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
3. Cómo usar Ultibro Breezhaler
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cantidad de Ultibro Breezhaler que debe usar
La dosis habitual es inhalar el contenido de una cápsula cada día.
Solo necesita inhalar el medicamento una vez al día ya que el efecto de este medicamento dura 24 horas. No use más dosis de la indicada por su médico.
Pacientes de edad avanzada (a partir de 75años de edad)
Si tiene 75 años o más puede usar este medicamento a la misma dosis que otros adultos.
Cuándo inhalar Ultibro Breezhaler
Use este medicamento en el mismo momento cada día. Esto le ayudará a recordar su uso.
Puede inhalar Ultibro Breezhaler en cualquier momento antes o después de alimentos o bebidas.
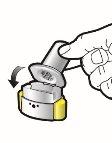
Cómo inhalar Ultibro Breezhaler
- Ultibro Breezhaler es para uso por vía inhalatoria.
- En este envase, encontrará un inhalador y cápsulas (en blísteres) que contienen el medicamento en polvo para inhalación. Utilice las cápsulas únicamente con el inhalador que se proporciona en este envase (inhalador de Ultibro Breezhaler). Las cápsulas deben mantenerse en el blíster hasta que necesite utilizarlas.
- Despegue la lámina del blíster para abrirlo - no presione la cápsula a través de la lámina.
- Cuando inicie un nuevo envase, use el nuevo inhalador de Ultibro Breezhaler que se proporciona en el envase.
- Deseche el inhalador de cada envase una vez que haya utilizado todas las cápsulas.
- No trague las cápsulas.
- Para más información acerca de cómo usar el inhalador, por favor lea las instrucciones al final de este prospecto.
Si usa másUltibro Breezhalerdel que debe
Si ha inhalado demasiado de este medicamento o si alguien usa sus cápsulas accidentalmente, informe a su médico inmediatamente o vaya al centro de urgencias más cercano. Muestre el envase de Ultibro Breezhaler. Puede ser necesaria atención médica. Puede notar que su corazón late más rápido de lo normal, o puede tener dolor de cabeza, sentirse somnoliento, sentir náuseas o tener que vomitar, o puede notar alteraciones visuales, estreñimiento o tener dificultad al orinar.
Si olvidó usarUltibro Breezhaler
Si olvidó inhalar una dosis en el horario habitual, inhálela lo más pronto posible en ese mismo día. Luego, el día siguiente inhale la dosis siguiente en el horario habitual. No inhale más de una dosis en el mismo día.
Cuánto tiempo debe continuar su tratamiento con UltibroBreezhaler
- Continúe usando Ultibro Breezhaler durante el tiempo que su médico le indique.
- La EPOC es una enfermedad de larga duración y usted debe utilizar Ultibro Breezhaler cada díay no solo cuando tenga problemas respiratorios u otros síntomas de EPOC.
Consulte a su médico o farmacéutico, si tiene dudas acerca de cuánto tiempo debe continuar su tratamiento con este medicamento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves:
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- dificultad para respirar o tragar, hinchazón de la lengua, labios o cara, urticaria, erupción cutánea – posibles síntomas de una reacción alérgica.
- sensación de cansancio o muy sediento, con aumento del apetito sin ganar peso y orina frecuente – posibles síntomas de un nivel elevado de azúcar en sangre (hiperglucemia).
Poco frecuentes (pueden afectar hasta 1 de cada 100pacientes)
- dolor opresor en el pecho con aumento de la sudoración – esto puede ser un problema grave de corazón (isquemia coronaria).
- hinchazón principalmente de la lengua, labios, cara o garganta (posibles signos de angioedema).
- dificultad para respirar con sibilancias o tos.
- dolor o molestias en los ojos, visión borrosa pasajera, halos visuales o imágenes coloreadas en asociación con un enrojecimiento de los ojos – posibles síntomas de glaucoma.
- latido irregular del corazón.
Si experimenta cualquiera de estos efectos adversos graves, busque ayuda médica inmediatamente.
Otros efectos adversos pueden incluir:
Muy frecuentes (pueden afectar a más de 1 de cada 10pacientes)
- nariz tapada, estornudos, tos, dolor de cabeza con o sin fiebre – posibles síntomas de una infección de las vías respiratorias superiores.
Frecuentes
- combinación de dolor de garganta y secreción nasal – posibles síntomas de rinofaringitis.
- micción frecuente y dolor al orinar – posibles síntomas de infección en las vías urinarias denominada cistitis.
- sensación de presión o dolor en mejillas y frente – posibles síntomas de inflamación de los senos denominada sinusitis.
- secreción o congestión nasal.
- mareo.
- dolor de cabeza.
- tos.
- dolor de garganta.
- estómago revuelto, indigestión.
- caries dental.
- dificultad y dolor al orinar – posibles síntomas de obstrucción de vejiga o de retención de orina.
- fiebre.
- dolor de pecho.
Poco frecuentes
- dificultad para dormir.
- latido rápido del corazón.
- palpitaciones – signos de latidos anómalos del corazón.
- alteración de la voz (ronquera).
- sangrado de nariz.
- diarrea o dolor de estómago.
- sequedad bucal.
- picor o erupción cutánea.
- dolor que afecta a los músculos, ligamentos, tendones, articulaciones y huesos.
- espasmo muscular.
- dolor muscular, dolor o sensibilidad.
- dolor en brazos o piernas.
- hinchazón de manos, tobillos y pies.
- cansancio.
Raros (pueden afectar hasta 1 de cada 1.000pacientes)
- hormigueo o entumecimiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ultibro Breezhaler
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el blíster después de «CAD»/«EXP». La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25°C.
Conservar las cápsulas en el blíster original para protegerlas de la humedad y no extraerlas hasta justo antes de usar.
El inhalador de cada envase debe desecharse una vez que haya utilizado todas las cápsulas.
No utilice este medicamento si observa que el envase está dañado o muestra indicios de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deUltibro Breezhaler
- Los principios activos son indacaterol (como maleato) y bromuro de glicopirronio. Cada cápsula contiene 143 microgramos de maleato de indacaterol equivalentes a 110 microgramos de indacaterol y 63 microgramos de bromuro de glicopirronio equivalentes a 50 microgramos de glicopirronio. La dosis liberada (la dosis que libera la boquilla del inhalador) es equivalente a 85 microgramos de indacaterol (equivalente a 110 microgramos de maleato de indacaterol) y 43 microgramos de glicopirronio (equivalente a 54 microgramos de bromuro de glicopirronio).
- Los demás componentes del polvo para inhalación son lactosa monohidrato y estearato de magnesio (ver sección 2 en el epígrafe “Ultibro Breezhaler contiene lactosa”).
- Los componentes de la cubierta de la cápsula son hipromelosa, cloruro de calcio, tartrazina (E102) y tinta de impresión negra (tapa) y azul (cuerpo).
- Los componentes de la tinta de impresión negra (tapa) son shellac (E904), propilenglicol, hidróxido de amonio, hidróxido de potasio y óxido de hierro negro (E172).
- Los componentes de la tinta de impresión azul (cuerpo) son shellac (E904), índigo carmín (E132) y dióxido de titanio (E171).
Aspecto de Ultibro Breezhaler y contenido del envase
Las cápsulas duras de Ultibro Breezhaler 85 microgramos/43 microgramos polvo para inhalación son transparentes y amarillas y contienen un polvo blanco o casi blanco. Tienen el código «IGP110.50» del producto impreso en azul bajo dos barras azules en el cuerpo y el logo de la compañía () impreso en negro en la tapa.
En este envase, podrá encontrar un dispositivo conocido como inhalador, junto con cápsulas en blisters. Cada blíster contiene 6 o 10 cápsulas duras.
Están disponibles los siguientes tamaños de envase:
Envase unitario conteniendo 6x1, 10x1, 12x1, 30x1 o 90x1 cápsulas duras, junto con 1 inhalador.
Envase múltiple conteniendo 96 (4 envases de 24x1) cápsulas duras y 4 inhaladores.
Envase múltiple conteniendo 150 (15 envases de 10x1) cápsulas duras y 15 inhaladores.
Envase múltiple conteniendo 150 (25 envases de 6x1) cápsulas duras y 25 inhaladores.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublín 4
Irlanda
Responsable de la fabricación
Novartis Farmacéutica SA
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom (Northern Ireland)Novartis Ireland Limited Tel: +44 1276 698370 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
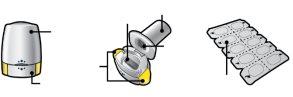
Lea las Instrucciones de Usocompletas antes de usar Ultibro Breezhaler. | |||
|
|
|
|
Introducir | Perforar y soltar |
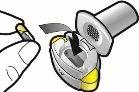
| Comprobar que la cápsula esté vacía |
|
|
| |
|
|
|
|
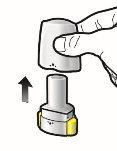
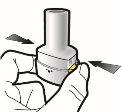
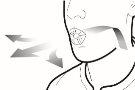
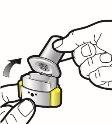
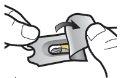
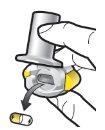
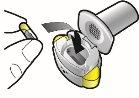
Paso 1a: Retire el capuchón | Paso 2a: Perfore la cápsula una sola vez Sujete el inhalador en posición vertical. Perfore la cápsula presionando firmemente ambos pulsadores al mismo tiempo. | Paso 3a: Espire completamente No sople dentro del inhalador. | Comprobar que la cápsula está vacía Abra el inhalador para comprobar si queda polvo en la cápsula. |
| Deberá oír un ruido cuando se perfore la cápsula. Perfore la cápsula sólo una vez. |
| Si queda polvo en la cápsula:
Queda polvoVacía |
Paso 1b: Abra el inhalador |
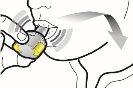
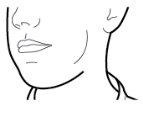
Paso 2b: Suelte completamente los pulsadores | Paso 3b: Inhale el medicamento profundamente Sujete el inhalador como se muestra en la figura. Introduzca la boquilla en su boca y cierre los labios firmemente en torno a ella. No presione los pulsadores. | |
| Inspire de forma rápida y tan profundamente como pueda. Durante la inhalación oirá un zumbido. Puede notar el gusto del medicamento cuando inhale. |
| |
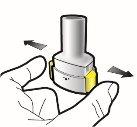
Paso 1c: Extraiga la cápsula Separe uno de los blísteres de la tira del blíster. Abra el blíster y extraiga una cápsula. No presione la cápsula a través de la lámina. No trague la cápsula. |
Paso 3c: Contenga la respiración Contenga la respiración durante 5 segundos | Extraiga la cápsula vacía Deseche la cápsula vacía en la basura de su casa. Cierre el inhalador y coloque de nuevo el capuchón. | |
Paso 1d: Introduzca la cápsula No coloque nunca la cápsula directamente en la boquilla. | Información importante
| ||
Paso 1e: Cierre el inhalador. | |||
Su envase de Ultibro Breezhaler contiene:
| Preguntas frecuentes ¿Por qué no hizo el inhalador un ruido al inhalar? La cápsula puede estar atascada en el compartimento. Si esto ocurre, libere la cápsula con cuidado, dando golpecitos en la base del inhalador. Inhale el medicamento de nuevo repitiendo los pasos 3a a 3c. Qué debo hacer si queda polvo en el interior de la cápsula? No ha recibido cantidad suficiente de su medicamento. Cierre el inhalador y repita los pasos 3a a 3c. He tosido después de inhalar, ¿es importante? Puede ocurrir. Si la cápsula está vacía, es que ha recibido suficiente cantidad de su medicamento. Noto pequeños fragmentos de la cápsula en mi lengua, ¿es importante? Puede ocurrir. No es perjudicial. La probabilidad de que las cápsulas se fragmenten aumenta si la cápsula se perfora más de una vez. | Limpieza del inhalador Frote la boquilla por dentro y por fuera con un paño limpio y seco, que no deje pelusa para eliminar cualquier residuo de polvo. Mantenga el inhalador seco. No lave nunca su inhalador con agua. | |
Eliminación del inhalador después de su uso Se debe desechar cada inhalador después de que todas las cápsulas se hayan usado. Pregunte a su farmacéutico cómo deshacerse de los medicamentos e inhaladores que ya no necesita. |
- País de registro
- Precio medio en farmacia70.25 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a Ultibro Breezhaler 85mcg/43mcg polvo para inhalacion (capsula dura)Forma farmacéutica: INHALACIÓN PULMONAR, 85 microgramos / 43 microgramosPrincipio activo: indacaterol and glycopyrronium bromideFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 85 MICROGRAMOS/43 MICROGRAMOSPrincipio activo: indacaterol and glycopyrronium bromideFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 55 MICROGRAMOS/22 MICROGRAMOSPrincipio activo: Vilanterol and umeclidinium bromideFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para Ultibro Breezhaler 85mcg/43mcg polvo para inhalacion (capsula dura)
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de Ultibro Breezhaler 85mcg/43mcg polvo para inhalacion (capsula dura), sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






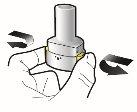
 Inhalar profundamente
Inhalar profundamente