TRUSOPT 20 mg/ml COLIRIO EN SOLUCION

Cómo usar TRUSOPT 20 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
TRUSOPT 20 mg/ml colirio en solución
dorzolamida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es TRUSOPT y para qué se utiliza
- Qué necesita saber antes de empezar a usar TRUSOPT
- Cómo usar TRUSOPT
- Posibles efectos adversos
- Conservación de TRUSOPT
- Contenido del envase e información adicional
1. Qué es TRUSOPT y para qué se utiliza
TRUSOPT contiene dorzolamida la cual pertenece a un grupo de medicamentos llamados "inhibidores de la anhidrasa carbónica".
Este medicamento se prescribe para bajar la presión elevada en el ojo y para tratar el glaucoma. Este medicamento puede ser utilizado solo o en combinación con otros medicamentos que disminuyen la presión en el ojo (llamados betabloqueantes).
2. Qué necesita saber antes de empezar a usar TRUSOPT
No use TRUSOPT
- si es alérgico a hidrocloruro de dorzolamida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene problemas o trastorno renal grave , o antecedentes de piedras en el riñón.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar TRUSOPT.
Informe a su médico o farmacéutico si tiene o ha tenido algún problema médico, incluyendo problemas oculares y cirugías oculares, y si tiene alguna alergia a cualquier medicación.
Si presenta cualquier irritación ocular o trastorno ocular nuevo, como enrojecimiento del ojo o hinchazón de los párpados, consulte inmediatamente a su médico.
Si sospecha que TRUSOPT le está causando una reacción alérgica (por ejemplo, erupción cutánea, reacción cutánea grave o prurito), deje de utilizar este medicamento y consulte inmediatamente a su médico.
Niños
TRUSOPT ha sido estudiado en lactantes y niños menores de 6 años de edad que presentaron presión elevada en el/los ojo(s) o habían sido diagnosticados con glaucoma. Para más información, consulte a su médico.
Pacientes de edad avanzada
En estudios con TRUSOPT, los efectos de este medicamento fueron semejantes tanto en los pacientes de edad avanzada como en los más jóvenes.
Uso en pacientes con deterioro hepático
Informe a su médico si sufre o ha sufrido problemas de hígado.
Uso de TRUSOPT con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento (incluyendo colirios). Esto es particularmente importante si está tomando otro inhibidor de la anhidrasa carbónica tal como la acetazolamida, o una sulfamida.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No deberá usar este medicamento durante el embarazo. Indique a su médico si está embarazada o tiene intención de estarlo.
Lactancia
Si se requiere el tratamiento con este medicamento, no se recomienda la lactancia. Indique a su médico si alimenta o pretende alimentar a su hijo mediante lactancia natural.
Conducción y uso de máquinas
No han sido realizados estudios de los efectos sobre la capacidad para conducir o utilizar máquinas. Hay efectos adversos asociados con TRUSOPT, tales como mareo y visión borrosa, que pueden afectar a su capacidad para conducir y/o manejar máquinas. No conducir o manejar máquinas hasta que se sienta bien o su visión sea clara.
TRUSOPT contiene cloruro de benzalconio
Este medicamento contiene aproximadamente 0,002 mg de cloruro de benzalconio en cada gota, equivalente a 0,075 mg/ml.
El cloruro de benzalconio se puede absorber por las lentes de contacto blandas alterando su color. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento.
3. Cómo usar TRUSOPT
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. La posología apropiada y la duración del tratamiento serán establecidas por su médico.
Cuando este medicamento es el único fármaco utilizado, la dosis recomendada es de una gota en el ojo u ojos afectado(s) por la mañana, tarde y noche.
Si su médico le ha recomendado que utilice este medicamento junto con un colirio betabloqueante para reducir la presión ocular, la dosis recomendada es de una gota de TRUSOPT en el ojo u ojos afectado(s) por la mañana y por la noche.
Si utiliza TRUSOPT con otro colirio, las gotas deben instilarse con un intervalo de tiempo mínimo de 10 minutos.
No deje que la punta del recipiente toque los ojos o las zonas que los rodean. Puede estar contaminada con bacterias capaces de causar infecciones oculares que originen graves daños en los ojos, e incluso la pérdida de la visión. Para evitar una posible contaminación del recipiente, lave sus manos antes de usar este medicamento y evite que la punta del recipiente entre en contacto con cualquier superficie. Si cree que su medicamento puede estar contaminado, o si desarrolla una infección ocular, consulte a su médico inmediatamente si puede seguir utilizando este envase.
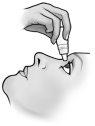
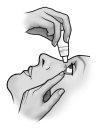
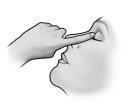
Instrucciones de uso
No utilice el envase si la tira de seguridad de plástico alrededor del cuello del envase no está o está rota. Cuando abra el envase por primera vez, arranque la tira de seguridad de plástico.
Cada vez que utilice TRUSOPT:
| |
| |
|
|
|
|
|
|
Si usa más TRUSOPT del que debe
Si se aplica demasiadas gotas en el ojo o traga algo del contenido del envase, debe avisar a su médico de inmediato.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono (91) 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar TRUSOPT
Es importante administrar este medicamento como le ha indicado su médico. Si olvida aplicar una dosis, debe administrársela lo antes posible. Sin embargo, si es casi la hora de la siguiente dosis, no se administre la dosis olvidada y continúe con el programa de dosis previsto normalmente.
No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con TRUSOPT
Si quiere interrumpir el uso de este medicamento, consulte primero con su médico. Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si desarrolla reacciones alérgicas tales como urticaria, hinchazón de la cara, labios, lengua y/o garganta que pueden causar dificultad en la respiración o al tragar, interrumpa el uso de este medicamento y consulte a su médico inmediatamente.
Los siguientes efectos adversos han sido comunicados con TRUSOPT durante los ensayos clínicos o tras la comercialización:
Efectos adversos muy frecuentes:(pueden afectar a más de 1 de cada 10 personas)
Quemazón y escozor de los ojos.
Efectos adversos frecuentes:(pueden afectar hasta 1 de cada 10 personas)
Enfermedad de la córnea con dolor de ojo y visión borrosa (queratitis punteada superficial), secreción con picor de los ojos (conjuntivitis), irritación/inflamación del párpado, visión borrosa, dolor de cabeza, náuseas, sabor amargo y fatiga.
Efectos adversos poco frecuentes:(pueden afectar hasta 1 de cada 100 personas)
Inflamación del iris.
Efectos adversos raros:(pueden afectar hasta 1 de cada 1.000 personas)
Hormigueo o entumecimiento de las manos o los pies, miopía transitoria que remite al cesar la terapia, desarrollo de fluido bajo la retina (desprendimiento coroideo, después de cirugía de filtración), dolor ocular, costras en el párpado, presión baja en el ojo, inflamación de la córnea (con síntomas de trastornos visuales), irritación ocular incluyendo enrrojecimiento, piedras en el riñón, mareos, hemorragia nasal, irritación de garganta, boca seca, erupción de la piel localizada (dermatitis de contacto), reacciones cutáneas graves, reacciones de tipo alérgico como erupción cutánea, urticaria, picor, en raros casos hinchazón de los labios, ojos y boca, falta de respiración, y más raramente dificultad para respirar.
Frecuencia no conocida:(no puede estimarse a partir de los datos disponibles)
Dificultad respiratoria, sensación de cuerpo extraño en el ojo (sensación de tener algo en el ojo), latidos cardiacos fuertes que pueden ser rápidos o irregulares (palpitaciones), aumento de la frecuencia cardiaca y aumento de la tensión arterial.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de TRUSOPT
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del envase y la caja después de ‘CAD’. La fecha de caducidad es el último día del mes que se indica.
TRUSOPT se debe utilizar dentro de un plazo de 28 días después de la apertura del envase.
Este medicamento no requiere ninguna temperatura especial de conservación. Conservar el envase en el embalaje exterior para protegerlo de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de TRUSOPT
- El principio activo es dorzolamida.
- Cada ml contiene 22,26 mg de hidrocloruro de dorzolamida equivalente a 20 mg de dorzolamida.
- Los demás componentes son hidroxietilcelulosa, manitol, citrato de sodio, hidróxido de sodio y agua para preparaciones inyectables. El cloruro de benzalconio se añade como conservante.
Aspecto del producto y contenido del envase
TRUSOPT es una solución clara, incolora a casi incolora, ligeramente viscosa.
TRUSOPT se presenta en un envase blanco translúcido con 5 ml de solución. El envase de plástico se cierra con un tapón de rosca blanco.
La tira de seguridad que se encuentra en la etiqueta del envase proporciona la evidencia de que el medicamento no ha sido manipulado inadecuadamente.
Tamaños de envases:
1 x 5 ml (un envase de 5 ml)
3 x 5 ml (tres envases de 5 ml)
6 x 5 ml (seis envases de 5 ml)
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finlandia
Responsable de la fabricación
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finlandia
Representante local
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Bélgica, Dinamarca, Finlandia, Francia, Alemania, Grecia, Irlanda, Italia, Países Bajos, Portugal, España y Suecia:
TRUSOPT
Fecha de la última revisión de este prospecto:06/2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia5.12 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TRUSOPT 20 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 20 mg/mlPrincipio activo: dorzolamidaFabricante: Tiedra Farmaceutica S.L.Requiere recetaForma farmacéutica: COLIRIO, 20 mg/mlPrincipio activo: dorzolamidaFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 2%Principio activo: dorzolamidaFabricante: Aristo Pharma Iberia S.L.Requiere receta
Médicos online para TRUSOPT 20 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TRUSOPT 20 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes