TOBRAMICINA ALTAN 300 MG/5 ML SOLUCION PARA INHALACION POR NEBULIZADOR


Cómo usar TOBRAMICINA ALTAN 300 MG/5 ML SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Tobramicina Altan300 mg/5 ml Solución para inhalación por nebulizador
Tobramicina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tobramicina Altan y para qué se utiliza
- Qué necesita saber antes de empezar a usar Tobramicina Altan
- Cómo usar Tobramicina Altan
- Posibles efectos adversos
- Conservación de Tobramicina Altan
- Contenido del envase e información adicional
1. Qué es Tobramicina Altan y para qué se utiliza
Tobramicina Altan contiene un medicamento denominado tobramicina, que es un antibiótico aminoglucósido.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas, como la gripe o el catarro. Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico. No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura. |
Para qué se utilizaTobramicina Altan
Tobramicina Altan se utiliza en pacientes a partir de 6 años de edad que padecen fibrosis quística, para el tratamiento de infecciones pulmonares causadas por una bacteria denominada Pseudomonas aeruginosa.
Tobramicina Altan combate la infección causada por la bacteria Pseudomonas en sus pulmones, y ayuda a mejorar su respiración.
Como funciona Tobramicina Altan
Cuando usted inhala Tobramicina Altan, el antibiótico puede llegar directamente a sus pulmones para combatir contra la bacteria que causa la infección. Para un mejor resultado de este medicamento, siga las instrucciones de este prospecto.
Qué esPseudomonas aeruginosa?
Es una bacteria muy común que infecta prácticamente a todos los pacientes que padecen fibrosis quística en algún momento de sus vidas. Algunos de ellos no cogen esta infección hasta un momento muy avanzado en sus vidas, mientras otros la padecen muy jóvenes.
Esta una de las bacterias más dañinas para personas con fibrosis quística. Si la infección no se controla adecuadamente, puede continuar dañando sus pulmones causando problemas adicionales a su respiración.
Tobramicina Altan mata la bacteria que causa infecciones en los pulmones. Esta infección se puede controlar con éxito si el problema se aborda en una fase temprana.
2. Qué necesita saber antes de empezar a usar Tobramicina Altan
No useTobramicina
- si es alérgico a tobramicina, a cualquier antibiótico aminoglucósido o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Si alguno de estos casos mencionados anteriormente le aplica, no tome este medicamento y consulte a su médico.
Consulte con su médico si cree que puede ser alérgico.
Advertencias y precauciones:
Consulte a su médico antes de empezar a usar Tobramicina si sufre o si alguna vez ha sufrido alguna de las siguientes condiciones:
- Problemas de audición (incluyendo zumbido de oídos y mareos).
- Problemas de riñón
- Dificultad inusual para respirar con sibilancias o tos, opresión en el pecho
- Sangre en su esputo (sustancia que expectora)
- Debilidad muscular que dura o empeora con el tiempo, síntomas mayoritariamente relacionados con la condición de miastenia o enfermedad de Parkinson.
Si alguno de estos casos le aplica, informe a su médico antes de usar Tobramicina
Si usted o los miembros de su familia materna tienen una enfermedad ocasionada por una mutación mitocondrial (una enfermedad genética) o pérdida de audición debido a antibióticos, se le recomienda informar a su médico o farmacéutico antes de tomar este medicamento. Ciertas mutaciones mitocondriales pueden aumentar su riesgo de pérdida auditiva con este producto. Su médico puede recomendar pruebas genéticas antes de la administración de tobramicia.
La inhalación de medicamentos puede causar opresión en el pecho y sibilancias y esto puede ocurrir con Tobramicina. Su médico supervisará su primera dosis de Tobramicina y controlará su función pulmonar antes y después de la dosis. Si usted no lo está haciendo, puede que su médico le haga utilizar un broncodilatador (p.ej. salbutamol), antes de usar Tobramicina.
Si usted está usando Tobramicina, las cepas de Pseudomonas pueden volverse con el tiempo resistentes al tratamiento. Esto significa que con el tiempo, el medicamento puede no funcionar tan bien como debería. Consulte con su médico si está preocupado por este tema.
Si la administración es mediante inyección, tobramicina puede causar en alguna ocasión pérdida de cabello, mareo y daño renal y puede perjudicar al feto.
Niños y adolescentes
Tobramicina se puede administrar a niños y adolescentes a partir de 6 años de edad. Tobramicina no se puede administrar a niños menores de 6 años.
Edad avanzada
Si usted tiene 65 años o es mayor de 65, su médico puede realizarle pruebas adicionales para decidir si Tobramicina es un tratamiento correcto para usted.
Uso de Tobramicina con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
No debe tomar los siguientes medicamentos mientras esté usando Tobramicina:
- Furosemida o ácido etacrínico, diuréticos
- Urea o manitol intravenoso
- Otros medicamentos que pueden dañar su sistema nervioso, riñones u oídos.
Los siguientes medicamentos pueden incrementar la aparición de efectos perjudiciales si se le administran mientras usted está recibiendo inyeccionesde tobramicina:
- Amfotericina B, cefalotina, ciclosporina, tacrolimus, polimixinas: estos medicamentos pueden dañar sus riñones.
- Compuestos de platino (tales como carboplatino y cisplatino): estos medicamentos pueden dañar sus riñones u oídos.
- Anticolinesterasas, (tales como neostigmina y piridostigmina), o toxina botulínica: estos medicamentos pueden causar la aparición o empeoramiento de debilidad muscular.
Si está tomando uno o más de los medicamentos descritos anteriormente, coméntelo con su médico antes de usar Tobramicina.
No debe mezclar ni diluir Tobramicina con ningún otro medicamento en su nebulizador.
Si usted está tomando varios tratamientos diferentes para la fibrosis quística, debe hacerlo en el siguiente orden:
- tratamiento broncodilatador, tal como salbutamol
- fisioterapia torácica
- otros medicamentos para inhalar
- Tobramicina. al final.
Compruebe así mismo este orden con su médico.
Embarazo y lactancia
Si usted está embarazada o quiere quedarse embarazada, consulte con su médico la posibilidad de que este medicamento pueda causarle algún daño a usted o al feto.
Se desconoce si al inhalar este medicamento cuando se está embarazada causa efectos adversos.
Cuando se administra mediante inyección, tobramicina y otros antibióticos aminoglucósidos pueden causar daño en un feto, tal como sordera.
Si usted está en periodo de lactancia, consulte a su médico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
Tobramicina no debe afectar a su capacidad para conducir y usar máquinas.
3. Cómo Cómo usar Tobramicina Altan
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Qué cantidad de este medicamento debe usar y cada cuánto lo debe usar
- La dosis recomendada es la misma para todas las personas a partir de 6 años.
- Utilice dosampollas cada día, durante 28 días. Inhale el contenido completo de una ampolla por la mañana y otra por la noche. Idealmente, debe haber un intervalo de 12 horas entre dosis.
- Debe dejar un periodo mínimo de 6 horasentre las dos inhalaciones de Tobramicina
- Después de tomar su medicamento durante 28 días, tendrá entonces un intervalo de 28 días en el cual no debe inhalar ninguna dosis de Tobramicina , antes de empezar otro ciclo.
- Es importante que mantenga el uso del producto dos veces cada día durante su periodo de tratamiento de 28 días y que mantenga los ciclos de 28 días de tratamiento, 28 días de descanso.
CON Tobramicina Altan | SIN Tobramicina Altan |
Tome Tobramicina Altan dos veces al día, cada día durante 28 días | No tome Tobramicina Altan durante los próximos 28 días |
Repita el ciclo
Si usa más Tobramicina del que debe
Si inhala demasiado Tobramicina la voz se le puede volver muy ronca. Asegúrese de informar a su médico tan pronto como sea posible. Si traga Tobramicina, informe a su médico tan pronto como sea posible.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Tobramicina
Si olvidó usar Tobramicina y faltan como mínimo 6 horas para la siguiente dosis, tome una nueva dosis lo antes posible. En caso contrario, espere a la siguiente dosis. No tome una dosis doble para compensar las dosis olvidadas.
Instrucciones para el uso de Tobramicina
Esta parte del prospecto explica cómo usar, cuidar y manejar Tobramicina. Lea y siga detalladamente estas instrucciones.
Si tiene alguna pregunta adicional sobre el uso de este medicamento, consulte a su médico o farmacéutico.
El equipo que necesita para inhalar Tobramicina
Tobramicina debe utilizarse con un nebulizador reutilizable, limpio y seco.
El nebulizador LC PLUS (fabricado por PARI GmbH) es adecuado para usar con Tobramicina.
Su médico o fisioterapeuta puede aconsejarle sobre el uso correcto de Tobramicina y el equipo que necesita. Puede necesitar diferentes nebulizadores para sus otros medicamentos inhalados para fibrosis quística.
Preparación de Tobramicina para inhalar
- Lave sus manos cuidadosamente con agua y jabón.
- Cada bolsa de aluminio de Tobramicina contiene una bandeja con 14 ampollas. Corte o rasgue la bolsa. Retire una ampolla de Tobramicina de la bandeja tirando suavemente de la lengüeta inferior para separarla del resto de ampollas. Vuelva a poner la bandeja en la bolsa de aluminio y guárdela en la nevera.
- Extienda las piezas de su nebulizador sobre una toalla de papel o de tela limpia y seca.
- Asegúrese que tiene el compresor adecuado y el tubo para conectar el nebulizador y el compresor.
- Siga con cuidado las instrucciones adecuadas para el uso de su modelo de nebulizador, debe leer el manual de instrucciones que el fabricante adjunta con el nebulizador. Compruebe que su nebulizador y compresor funcionan adecuadamente de acuerdo con las instrucciones del fabricante antes de empezar a usar su medicamento.
Uso de Tobramicina con LCPLUS(PARI GmbH)
Para instrucciones más detalladas sobre el uso y cuidado del nebulizador, lea el manual de instrucciones que se adjunta con el PARI LC PLUS.
- Retire la parte superior del nebulizador haciéndola girar en sentido contrario a las agujas del reloj y levantándola. Coloque dicha parte superior sobre la toalla y ponga el cartucho del nebulizador de pie sobre la toalla.
- Conecte uno de los extremos del tubo a la salida de aire del compresor. Asegúrese de que el tubo ajusta perfectamente. Conecte el compresor a la toma de corriente.
- Abra la ampolla de Tobramicina sujetando la lengüeta inferior con una mano y separando con la otra mano la punta superior, torciéndola. Vacíe todo el contenido de la ampolla en el cartucho del nebulizador.
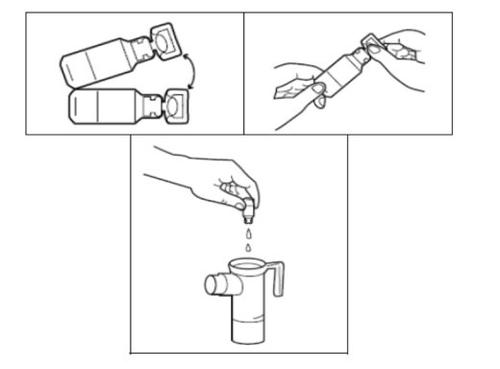
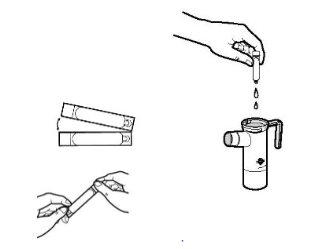
- Vuelva a colocar la parte superior del nebulizador, fije la boquilla y coloque la tapa de la válvula de inspiración en el nebulizador. Conecte entonces el compresor tal y como se indica en el manual de instrucciones de su nebulizador PARI LC PLUS.
- Encienda el compresor. Verifique que haya una neblina constante saliendo de la boquilla. Si no hay neblina, controle todas las conexiones del tubo y si está funcionando debidamente el compresor.
- Siéntese o póngase de pie, de manera que pueda respirar normalmente
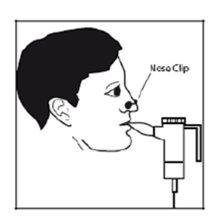
- Coloque la boquilla entre sus dientes y sobre la punta de la lengua. Respire normalmente, pero sólo por la boca (posiblemente le sea útil la pinza para la nariz). Trate de no bloquear el flujo del aire con la lengua.
- Continúe hasta que todo el Tobramicina Altan se haya consumido y no se produzca más neblina. La inhalación completa debe durar alrededor de 15 minutos. Es posible que escuche un sonido de gorgoteo cuando el recipiente del nebulizador esté vacío.
- Acuérdese de limpiar y desinfectar su nebulizador después del tratamiento de acuerdo con el manual de instrucciones del fabricante. No debe usar nunca un nebulizador sucio u obstruido. No debe compartir su nebulizador con otras personas.
Si le interrumpen, o si necesita toser o descansar durante la administración, apague el compresor para no desperdiciar el medicamento.
Vuelva a encender el compresor cuando esté usted preparado para reiniciar el tratamiento. Omita esta dosis si su próxima dosis le corresponde en menos de 6 horas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves
Si sufre cualquiera de los efectos adversos siguientes, interrumpa el uso de Tobramicina e informe a su médico inmediatamente:
- Dificultad inusual para respirar con sibilancias o tos y opresión en el pecho
- Reacciones alérgicas incluyendo urticaria y picor
Si sufre cualquiera de los efectos adversos siguientes, informe a su médico inmediatamente:
- Pérdida de audición (el zumbido en los oídos es una señal de peligro potencial de pérdida de audición), ruidos (como silbidos) en los oídos
Su enfermedad pulmonar de base puede empeorar mientras esté usando Tobramicina. Esto puede ser debido a falta de eficacia. Informe a su médico inmediatamente si esto ocurre.
Algunos efectos adversos son muy frecuentes
Estos efectos adversos pueden afectar a más de 1 de cada 10 personas.
- Goteo o congestión nasal, estornudos
- Alteración de la voz (ronquera)
- Decoloración de la sustancia que expectora (esputo)
- Empeoramiento de los resultados de las pruebas de la función pulmonar
Si alguno de ellos le afecta gravemente, informe a su médico.
Algunos efectos adversos son frecuentes
Estos efectos adversos pueden afectar hasta 1 de cada 10 personas.
- Sensación de malestar general
- Dolor muscular
- Alteración de la voz con dolor de garganta y dificultad para tragar (laringitis)
Si alguno de ellos le afecta gravemente, informe a su médico.
Otros efectos adversos:
- Picor
- Erupción cutánea con picor
- Erupción cutánea
- Pérdida de la voz
- Sentido del gusto alterado
- Dolor de garganta
Si alguno de ellos le afecta gravemente, informe a su médico.
Si usted ha recibido Tobramicina al mismo tiempo o después de ciclos repetidos de tobramicina u otros antibióticos aminoglucósidos inyectados, se ha notificado pérdida de audición como efecto secundario.
Las inyecciones de tobramicina u otros aminoglucósidos pueden causar reacciones alérgicas, problemas de audición y problemas renales.
Los pacientes con fibrosis quística presentan varios síntomas propios de la enfermedad. Estos pueden incluso presentarse mientras se está tomando Tobramicina pero no deberían ser más frecuentes o parecer peores que antes del tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano (www.notificaRAM.es). Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tobramicina Altan
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase, en la bolsa, o impreso en la ampolla.
- No utilice este medicamento si observa que se vuelve turbio o si hay partículas en la solución.
- Conservar en nevera(entre 2ºC y 8ºC). Si usted no dispone de nevera (por ejemplo durante el transporte del medicamento) puede mantener las bolsas de aluminio (abiertas o cerradas) a temperatura ambiente (no superior a 25°C) durante un tiempo máximo de 28 días.
- No utilice las ampollas de Tobramicina si las ha conservado a temperatura ambiente durante más de 28 días.
- Conservar la ampolla en el embalaje original, para protegerlo de la luz. Este medicamento suele presentar un color amarillo claro, que puede variar y algunas veces puede ser amarillo oscuro. Esto no afecta a la actividad de este medicamento, siempre que se sigan las instrucciones de conservación.
No debe conservar jamás una ampolla abierta. Una vez abierta una ampolla debe usarse inmediatamente y cualquier resto de producto debe ser desechado.
6. Contenido del envase e información adicional
Composición de Tobramicina Altan
- El principio activo es tobramicina. Una ampolla contiene 300 mg de tobramicina como dosis única.
- Los demás componentes son cloruro de sodio, agua para preparaciones inyectables, hidróxido de sodio y ácido sulfúrico (para ajustar el nivel de acidez).
Aspecto del producto y contenido del envaseTobramicina Altan es una solución transparente, ligeramente amarilla que se presenta en una ampolla lista para usar.
Las ampollas están envasadas en bolsas de aluminio, una bolsa de aluminio contiene 7 ampollas que corresponden a 7 días de tratamiento.
Tobramicina Altan está disponible en envases de 56 ampollas, los cuales son suficientes para un ciclo ciclos de tratamiento.
Puede que todos los tamaños de envases no estén comercializados.
Titular de la autorización de comercialización:
Altan Pharmaceuticals, S.A.
C/ Cólquide, Nº 6, Portal 2, 1ª Planta, Oficina F.
Edificio Prisma,
Las Rozas, 28230 Madrid
Responsable de la fabricación:
Altan Pharmaceuticals, S.A.
Polígono Industrial de Bernedo, s/n
01118 Bernedo (Álava)
Spain
Altan Pharmaceuticals, S.A.
Avda. de la Constitución, 198-199
Polígono Industrial Monte Boyal, Casarrubios del Monte, 45950 Toledo
Spain
Este medicamento está autorizado en los siguientes estados miembros de la UE con los siguientes nombres:
France:Tobramycine Zentiva 300 mg/5 ml Solution pour inhalation par nébuliseur
Germany: Tobramycin Zentiva 300 mg/ 5 ml Lösung für einen Vernebler
Italy: Tobramicina Altan 300 mg/5 ml soluzione per nebulizzatore
Portugal: Tobramicina Altan. 300 mg/ 5 ml Solução para Inalação por Nebulização
Spain: Tobramicina Altan. 300 mg/ 5 ml Solución para inhalación por nebulizador
United Kingdom: Tobramycin 300 mg/ 5 ml Nebuliser solution
Fecha de la última revisión de este prospecto: octubre 2023
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/”
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TOBRAMICINA ALTAN 300 MG/5 ML SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INHALACIÓN PULMONAR, 300 mg / 4 mlPrincipio activo: TobramicinaFabricante: Chiesi España S.A.U.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 28 mg tobramicinaPrincipio activo: TobramicinaFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 300 mg/5 mlPrincipio activo: TobramicinaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para TOBRAMICINA ALTAN 300 MG/5 ML SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TOBRAMICINA ALTAN 300 MG/5 ML SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes