SUBOXONE 8 MG/2 MG COMPRIMIDOS SUBLINGUALES


Cómo usar SUBOXONE 8 MG/2 MG COMPRIMIDOS SUBLINGUALES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Suboxone 8 mg/2 mg comprimidos sublinguales
buprenorfina/naloxona
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos signos de enfermedad que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Suboxone y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Suboxone
- Cómo tomar Suboxone
- Posibles efectos adversos
- Conservación de Suboxone
- Contenido del envase e información adicional
1. Qué es Suboxone y para qué se utiliza
Suboxone se utiliza para tratar la dependencia de opioides (narcóticos), como la heroína o la morfina, en drogadictos que han dado su conformidad para ser tratados por su adicción. Suboxone se utiliza en adultos y adolescentes mayores de 15 añosque también están recibiendo apoyo médico, social y psicológico.
2. Qué necesita saber antes de empezar a tomar Suboxone
No tome Suboxone
- si es alérgico a buprenorfina, naloxona o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si padece problemas respiratorios graves;
- si padece problemas graves de hígado;
- si sufre una intoxicación etílicao si padece temblores, sudoración, ansiedad, confusión o alucinaciones causadas por el alcohol;
- si está tomando naltrexonao nalmefenopara el tratamiento de la dependencia del alcohol o de opioides.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar Suboxone si tiene:
- asma u otros problemas respiratorios;
- problemas de hígado como hepatitis;
- tensión arterial baja;
- una lesión traumática en la cabeza o una enfermedad cerebral recientes;
- un trastorno urinario (especialmente asociado a un aumento del tamaño de la próstata en hombres);
- una enfermedad de riñón;
- problemas de tiroides;
- un trastorno de la corteza suprarrenal (p. ej., enfermedad de Addison);
- depresión u otras enfermedades que se tratan con antidepresivos. El uso de estos medicamentos junto con Suboxone puede provocar síndrome serotoninérgico, una enfermedad potencialmente mortal (ver “Otros medicamentos y Suboxone”).
Aspectos importantes que se deben tener en cuenta:
- En caso de ingestión accidental o sospecha de ingestión, se debe poner en contacto inmediatamente con una unidad de urgencias.
- Control adicional
Si tiene más de 65 años, puede que su médico le controle más estrechamente.
- Uso indebido y abuso
Este medicamento puede ser un objetivo para las personas que abusan de los medicamentos con receta y debe mantenerse en un lugar seguro para protegerlo del robo (ver sección 5). No dé estemedicamento a ninguna otra persona. Puede causarle la muerte u otro tipo de daños.
- Problemas respiratorios
Algunas personas han fallecido por insuficiencia respiratoria (incapacidad para respirar) porque han utilizado buprenorfina de forma indebida o la tomaron junto con otros depresores del sistema nervioso central, como el alcohol, las benzodiazepinas (tranquilizantes) u otros opioides.
Este medicamento puede provocar depresión respiratoria (dificultad para respirar) grave, posiblemente mortal, en niños y personas no dependientes si lo ingieren de forma accidental o intencionada.
- Dependencia
Este medicamento puede causar dependencia.
- Síntomas de abstinencia
Este medicamento puede causar síntomas de abstinencia a los opioides si lo toma demasiado pronto después de usar opioides. Debe dejar que transcurran al menos 6 horas después de usar un opioide de acción corta (p. ej., morfina, heroína) o al menos 24 horas después de usar un opioide de acción prolongada, como la metadona.
Este medicamento también puede causar síntomas de abstinencia si deja de tomarlo de manera repentina. Ver sección 3 “Si interrumpe el tratamiento”.
- Afectación del hígado
Se han descrito casos de afectación del hígado después de tomar Suboxone, especialmente cuando se hace un uso indebido del medicamento. Podría deberse asimismo a infecciones víricas (p. ej., hepatitis C crónica), al abuso de alcohol, a la anorexia o al uso de otros medicamentos que pueden dañar el hígado (ver sección 4). Su médico puede realizarleanálisis de sangre frecuentes para controlar el estado de su hígado. Informe a su médico si tiene algún problema de hígado antes de comenzar el tratamiento con Suboxone.
- Tensión arterial
Este medicamento puede causar una bajada repentina de la tensión arterial, haciendo que se sienta mareado si se levanta demasiado rápido después de estar sentado o acostado.
- Diagnóstico de afecciones médicas no relacionadas
Este medicamento puede enmascarar los síntomas de dolor que podrían ayudar en el diagnóstico de algunas enfermedades. Debe informar a su médico que toma este medicamento.
Niños y adolescentes
Nodé este medicamento a niños menores de 15 años. Si tiene entre 15 y 18 años, su médico puede controlarle más estrechamente durante el tratamiento, debido a la falta de datos en este grupo de edad.
Otros medicamentos y Suboxone
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Algunos medicamentos pueden aumentar los efectos adversos de Suboxone, y pueden ser graves. No tome otros medicamentos al mismo tiempo que Suboxone sin consultar antes a su médico, especialmente:
- Benzodiazepinas(utilizadas para tratar la ansiedad o los trastornos del sueño) como, por ejemplo, diazepam, temazepam o alprazolam. El uso concomitante de Suboxone y sedantes como las benzodiazepinas o medicamentos relacionados aumenta el riesgo de somnolencia, dificultad para respirar (depresión respiratoria) y coma, y podría ser potencialmente mortal. Por este motivo, solo se debe considerar el uso concomitante cuando otras opciones de tratamiento no son posibles. Sin embargo, si su médico le receta Suboxone junto con sedantes, su médico debe limitar la dosis y la duración del tratamiento concomitante. Informe a su médico sobre todos los sedantes que toma y siga estrictamente la recomendación posológica de su médico. Podría resultar útil informar a sus amigos o familiares para que estén alerta a los signos y síntomas anteriormente indicados. Póngase en contacto con su médico si presenta estos síntomas.
- Otros medicamentos que pueden provocar somnolencia y quese emplean para el tratamiento de enfermedades como la ansiedad, el insomnio, las convulsiones/crisis epilépticas o el dolor. Este tipo de medicamentos pueden disminuir su nivel de alerta, dificultando así la conducción y el uso de máquinas. También pueden causar depresión del sistema nervioso central, lo que es muy grave. A continuación se incluye una lista con ejemplos de este tipo de medicamentos:
- otros medicamentos que contienen opioides como la metadona, algunos analgésicos o antitusígenos;
- los antidepresivos (utilizados para el tratamiento de la depresión) como isocarboxazida, fenelzina, selegilina, tranilcipromina y valproato pueden potenciar los efectos de este medicamento;
- antagonistas de los receptores H1 sedantes (utilizados para el tratamiento de reacciones alérgicas) como difenhidramina y clorfenamina;
- barbitúricos (utilizados para producir sueño o sedación) como fenobarbital o secobarbital;
- tranquilizantes (utilizados para producir sueño o sedación) como hidrato de cloral.
- Antidepresivoscomo moclobemida, tranilcipromina, citalopram, escitalopram, fluoxetina, fluvoxamina, paroxetina, sertralina, duloxetina, venlafaxina, amitriptilina, doxepina o trimipramina. Estos medicamentos pueden interactuar con Suboxone y puede experimentar síntomas como contracciones musculares rítmicas involuntarias, incluidos los músculos que controlan el movimiento de los ojos, agitación, alucinaciones, coma, sudoración excesiva, temblores, exageración de los reflejos, aumento de la tensión muscular, temperatura corporal superior a 38 °C. Póngase en contacto con su médico si sufre estos síntomas.
- La clonidina (utilizada para el tratamiento de la tensión arterial alta) puede alargar los efectos de este medicamento.
- Los antirretrovirales (utilizados para el tratamiento del VIH) como ritonavir, nelfinavir o indinavir pueden potenciar los efectos de este medicamento.
- Determinados antifúngicos (utilizados para el tratamiento de infecciones por hongos) como ketoconazol, itraconazol o ciertos antibióticos pueden alargar los efectos de este medicamento.
- Algunos medicamentos pueden disminuir el efecto de Suboxone. Estos incluyen los medicamentos empleados para el tratamiento de la epilepsia (carbamazepina y fenitoína) y los medicamentos utilizados para tratar la tuberculosis (rifampicina).
- La naltrexona y el nalmefeno (medicamentos utilizados para tratar trastornos adictivos) pueden bloquear los efectos terapéuticos de Suboxone. No se deben tomar al mismo tiempo que el tratamiento con Suboxone puesto que puede experimentar un inicio repentino de abstinencia prolongada e intensa.
Uso de Suboxone con alimentos, bebidas y alcohol
No tome alcoholmientras está recibiendo tratamiento con este medicamento. El alcohol puede aumentar la somnolencia y el riesgo de insuficiencia respiratoria si se toma con Suboxone. No trague ni consuma alimentos o bebidas hasta que el comprimido se haya disuelto por completo.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Se desconocen los riesgos del uso de Suboxone en mujeres embarazadas. Su médico decidirá si debe continuar su tratamiento con otro medicamento.
Cuando se toman durante el embarazo, especialmente en los últimos meses, los medicamentos como Suboxone pueden dar lugar a síntomas de abstinencia del medicamento, incluidos problemas respiratorios, en el recién nacido. Esto puede ocurrir varios días después del parto.
No dé el pecho mientras toma este medicamento, ya que buprenorfina se excreta en la leche materna.
Conducción y uso de máquinas
Noconduzca ni monte en bicicleta, no use herramientas ni máquinas, ni realice actividades peligrosas hasta que sepa cómo le afecta este medicamento. Suboxone puede causar somnolencia, mareo o alteración del pensamiento. Esto puede ocurrir con mayor frecuencia en las primeras semanas de tratamiento, cuando la dosis se está cambiando, pero también puede ocurrir si bebe alcohol o si toma otros medicamentos sedantes al mismo tiempo que toma Suboxone.
Suboxone contiene lactosa y sodio.
Este medicamento contiene lactosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Suboxone
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
El tratamiento lo recetan y supervisan médicos con experiencia en el tratamiento de la drogadicción.
Su médico determinará cuál es la mejor dosis para usted. Durante el tratamiento, el médico puede ajustarle la dosis, en función de su respuesta al tratamiento.
Inicio del tratamiento
La dosis inicial recomendada en adultos y adolescentes mayores de 15 años es normalmente de dos comprimidos sublinguales de Suboxone 2 mg/0,5 mg.
Esta dosis se puede repetir otras dos veces el día 1, en función de sus necesidades.
Antes de tomar su primera dosis de Suboxone, debe conocer los síntomas claros de abstinencia. Su médico le dirá cuándo debe tomar su primera dosis.
- Inicio del tratamiento con Suboxone si tiene dependencia de la heroína
Si tiene dependencia de la heroína o de un opioide de acción corta, la primera dosis debe tomarla cuando aparezcan los signos de abstinencia, al menos 6 horas después de la últimavez que usara opioides.
- Inicio del tratamiento con Suboxone si tiene dependencia de la metadona
Si ha estado tomando metadona o un opioide de acción prolongada, lo ideal es reducir la dosis de metadona a menos de 30 mg/día antes de comenzar el tratamiento con Suboxone. La primera dosis de Suboxone debe tomarla cuando aparezcan signos de abstinencia y al menos 24 horasdespués de la última vez que usara metadona.
Cómo tomar Suboxone
- Tome la dosis una vez al día, poniendo los comprimidos debajo de la lengua.
- Mantenga los comprimidos debajo de la lengua hasta que se hayan disuelto por completo. Esto puede tardar entre 5 y 10 minutos.
- No mastique ni trague los comprimidos, puesto que el medicamento no funcionará y puede presentar síntomas de abstinencia.
No consuma alimentos ni bebidas hasta que los comprimidos se hayan disuelto por completo.
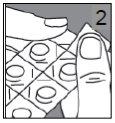
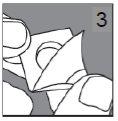
Cómo sacar el comprimido del blíster
|
|
|
|
|
|
Si el blíster está dañado, tire el comprimido.
Ajuste de la dosis y tratamiento de mantenimiento:
Durante los días posteriores al comienzo del tratamiento, su médico puede aumentarle la dosis de Suboxone que toma según sus necesidades. Si usted cree que el efecto de Suboxone es demasiado intenso o demasiado débil, informe a su médico o farmacéutico. La dosis diaria máxima es de 24 mgde buprenorfina.
Después de un tiempo de tratamiento satisfactorio, puede acordar con su médico la disminución gradual de la dosis a una dosis de mantenimiento más baja.
Interrupción del tratamiento
En función de su estado, la dosis de Suboxone puede seguir disminuyéndose bajo una supervisión médica estrecha, hasta que finalmente pueda llegar a interrumpirse.
No cambie el tratamiento de ninguna manera ni lo interrumpa sin la autorización del médico que le está tratando.
Si toma más Suboxone del que debe
Si usted, u otra persona, toma una cantidad excesiva de este medicamento, debe ir o le deben llevar inmediatamente a Urgencias o a un hospital para recibir tratamiento, ya que la sobredosisde Suboxone puede causar problemas respiratorios graves y potencialmente mortales.
Los síntomas de sobredosis pueden incluir una sensación de somnolencia y descoordinación con reflejos lentos, visión borrosa y/o dificultad para hablar. Puede que no sea capaz de pensar con claridad y que respire mucho más lento de lo que es normal para usted.
Si olvidó tomar Suboxone
Si olvida tomar una dosis, informe a su médico lo antes posible.
Si interrumpe el tratamiento con Suboxone
No cambie el tratamiento de ninguna manera ni lo interrumpa sin la autorización del médico que le está tratando. La interrupción repentina del tratamiento puede causar síntomas de abstinencia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe inmediatamente a su médico o busque atención médica urgentesi presenta efectos adversos como:
- Hinchazón de la cara, los labios, la lengua o la garganta, que puede causar dificultad para tragar o respirar; habón urticarial/urticaria intensos. Estos pueden ser signos de una reacción alérgica potencialmente mortal.
- Sensación de somnolencia y descoordinación, visión borrosa, dificultad para hablar, incapacidad para pensar con claridad o respiración mucho más lenta de lo que es normal para usted.
Asimismo, informe inmediatamente a su médicosi presenta efectos adversos como:
- Cansancio intenso, picor con coloración amarillenta de la piel o los ojos. Estos pueden ser signos de afectación del hígado.
- Ver u oír cosas que en realidad no existen (alucinaciones).
Efectos adversos comunicados con Suboxone
Efectos adversos muy frecuentes (pueden afectar a más de una de cada 10 personas): |
Insomnio (incapacidad para dormir), estreñimiento, náuseas, sudoración excesiva, dolor de cabeza, síndrome de abstinencia de fármacos. |
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas): |
Pérdida de peso, hinchazón de manos y pies, somnolencia, ansiedad, nerviosismo, hormigueo, depresión, disminución del deseo sexual, aumento de la tensión muscular, pensamiento anormal, aumento del lagrimeo (ojos llorosos) u otro trastorno del lagrimeo, visión borrosa, sofocos, aumento de la tensión arterial, migrañas, moqueo, dolor de garganta y dolor al tragar, aumento de la tos, malestar de estómago u otras molestias en el estómago, diarrea, anomalías de la función hepática, flatulencia, vómitos, erupción cutánea, picazón, habón urticarial, dolor, dolor articular, dolor muscular, calambres en la pierna (espasmo muscular), dificultad para conseguir o mantener una erección, anormalidad de la orina, dolor abdominal, dolor de espalda, debilidad, infección, escalofríos, dolor torácico, fiebre, síntomas de tipo gripal, sensación de malestar general, lesión accidental debida a la pérdida del estado de alerta o la coordinación, desvanecimiento y mareo. |
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas): |
Glándulas inflamadas (ganglios linfáticos), agitación, temblor, sueños anormales, actividad muscular excesiva, despersonalización (no sentirse como uno mismo), dependencia del medicamento, amnesia (trastorno de la memoria), pérdida de interés, sensación exagerada de bienestar, convulsión (crisis epilépticas), trastorno del habla, pupilas de pequeño tamaño, dificultad para orinar, inflamación o infección de los ojos, latido cardiaco rápido o lento, tensión arterial baja, palpitaciones, ataque al corazón, opresión en el pecho, falta de aliento, asma, bostezos, dolor y llagas en la boca, decoloración de la lengua, acné, nódulo en la piel, pérdida del pelo, piel seca o descamada, inflamación articular, infección del tracto urinario, análisis de sangre anormales, sangre en la orina, eyaculación anormal, problemas menstruales o vaginales, piedra en el riñón, proteína en la orina, dolor o dificultad al orinar, sensibilidad al calor o al frío, golpe de calor, pérdida de apetito, sentimientos de hostilidad. |
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): |
Síndrome de abstinencia repentino causado por tomar Suboxone demasiado pronto después del consumo de opioides ilegales, síndrome de abstinencia de fármacos en los recién nacidos, respiración lenta o dificultad para respirar, daño hepático con o sin ictericia, alucinaciones, hinchazón de la cara y la garganta o reacciones alérgicas potencialmente mortales, bajada de la tensión arterial al cambiar de posición de estar sentado o acostado a estar de pie. |
El mal uso de este medicamento mediante inyección puede causar síntomas de abstinencia, infecciones, otras reacciones cutáneas y problemas de hígado potencialmente graves (ver “Advertencias y precauciones”).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Suboxone
Mantener este medicamento fuera de la vista y del alcance de los niños y otros miembros de la familia.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación. Sin embargo, Suboxone puede ser un objetivo para las personas que abusan de los medicamentos con receta. Mantenga este medicamento en un lugar seguro para protegerlo del robo.
Guardar el blíster de forma segura.
No abrir nunca el blíster antes de tiempo.
No tome este medicamento delante de los niños.
En caso de ingestión accidental o sospecha de ingestión, se debe poner en contacto inmediatamente con una unidad de urgencias.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Suboxone
- Los principios activos son buprenorfina y naloxona.
Cada comprimido sublingual de 8 mg/2 mg contiene 8 mg de buprenorfina (como clorhidrato) y 2 mg de naloxona (como clorhidrato dihidratado).
- Los demás componentes son lactosa monohidrato, manitol, almidón de maíz, povidona K30, ácido cítrico anhidro, citrato de sodio, estearato de magnesio, acesulfamo de potasio y aroma natural de lima y limón.
Aspecto del producto y contenido del envase
Suboxone 8 mg/2 mg comprimidos sublinguales son comprimidos blancos, hexagonales y biconvexos
Envasados en envases de 7 y 28 comprimidos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Indivior Europe Limited
27 Windsor Place
Dublin 2
Irlanda
Responsable de la fabricación
Reckitt Benckiser Healthcare (UK) Ltd,
Dansom Lane,
Hull,
East Yorkshire HU8 7DS,
Reino Unido
O
Indivior Europe Limited
27 Windsor Place
Dublin 2
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Indivior Europe Limited Tél/Tel: 0800 780 41 e-mail: [email protected] | Lietuva Indivior Europe Limited Tel: 880030793 e-mail: [email protected] |
???????? Indivior Europe Limited Te?.: 00800 110 4104 e-mail: [email protected] | Luxembourg/Luxemburg Indivior Europe Limited Tél/Tel: 800 245 43 e-mail: [email protected] |
Ceská republika Indivior Europe Limited Tel: 800 143 737 e-mail: [email protected] | Magyarország Indivior Europe Limited Tel.: 6800 19301 e-mail: [email protected] |
Danmark Indivior Europe Limited Tlf: 80826653 e-mail: [email protected] | Malta Indivior Europe Limited Tel: 80062185 e-mail: [email protected] |
Deutschland Indivior Europe Limited Tel: 800 181 3799 e-mail: [email protected] | Nederland Indivior Europe Limited Tel: 0800 022 87 83 e-mail: [email protected] |
Eesti Indivior Europe Limited Tel: 8000041004 e-mail: [email protected] | Norge Indivior Europe Limited Tlf: 80016773 e-mail: [email protected] |
Ελλ?δα Indivior Europe Limited Τηλ: 800 270 81 901 e-mail: [email protected] | Österreich Indivior Europe Limited Tel: 800 296551 e-mail: [email protected] |
España Indivior Europe Limited Tel: 900 994 121 e-mail: [email protected] | Polska Indivior Europe Limited Tel.: 0800 4111237 e-mail: [email protected] |
France Indivior Europe Limited Tél:0800 909 972 e-mail: [email protected] | Portugal Indivior Europe Limited Tel: 800 841 042 e-mail: [email protected] |
Hrvatska Indivior Europe Limited Tel: + +0800 222 899 e-mail: [email protected] | România Indivior Europe Limited Tel: 800 477 029 e-mail: [email protected] |
Ireland Indivior Europe Limited Tel: 1800554156 e-mail: [email protected] | Slovenija Indivior Europe Limited Tel: 080080715 e-mail: [email protected] |
Ísland Indivior Europe Limited Sími: 8009875 Netfang: [email protected] | Slovenská republika Indivior Europe Limited Tel: 800110286 e-mail: [email protected] |
Italia Indivior Europe Limited Tel: 800 789 822 e-mail: [email protected] | Suomi/Finland Indivior Europe Limited Puh/Tel: 0800417489 e-mail: [email protected] |
Κ?προς Indivior Europe Limited Τηλ: 800 270 81 901 e-mail: [email protected] | Sverige Indivior Europe Limited Tel: 020791680 e-mail: [email protected] |
Latvija Indivior Europe Limited Tel: 800 05612 e-mail: [email protected] | United Kingdom Indivior Europe Limited Tel: 0808 234 9243 e-mail: [email protected] |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SUBOXONE 8 MG/2 MG COMPRIMIDOS SUBLINGUALESForma farmacéutica: COMPRIMIDO SUBLINGUAL, 2 mg/0,5 mgPrincipio activo: buprenorphine, combinationsFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 8 mg/2 mgPrincipio activo: buprenorphine, combinationsFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 12 mg/3 mgPrincipio activo: buprenorphine, combinationsFabricante: Indivior Europe LimitedRequiere receta
Médicos online para SUBOXONE 8 MG/2 MG COMPRIMIDOS SUBLINGUALES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SUBOXONE 8 MG/2 MG COMPRIMIDOS SUBLINGUALES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes