
STREFEN SPRAY 8,75 MG/DOSIS SOLUCION PARA PULVERIZACION BUCAL SABOR MIEL Y LIMON

Cómo usar STREFEN SPRAY 8,75 MG/DOSIS SOLUCION PARA PULVERIZACION BUCAL SABOR MIEL Y LIMON
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Strefen Spray 8,75 mg/dosis solución para pulverización bucalsabor miel y limón
flurbiprofeno
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 3 días.
Contenido del prospecto
- Qué es Strefen Spray y para qué se utiliza.
- Qué necesita saber antes de empezar a usar Strefen Spray.
- Cómo usar Strefen Spray.
- Posibles efectos adversos.
- Conservación de Strefen Spray.
- Contenido del envase e información adicional.
1. Qué es Strefen Spray y para qué se utiliza
El principio activo es flurbiprofeno. El flurbiprofeno pertenece a un grupo de medicamentos denominados antiinflamatorios no esteroideos (AINE) que actúan modificando la respuesta del organismo ante el dolor, la inflamación y la fiebre.
Strefen Spray se utiliza para el alivio de los síntomas del dolor de garganta agudo tales como irritación, dolor, dificultad para tragar e inflamación en adultos mayores de 18 años.
2. Qué necesita saber antes de usar Strefen Spray
No use Strefen Spray:
- Si es alérgico al flurbiprofeno, a otros antiinflamatorios no esteroideos (AINE), a la aspirina (ácido acetilsalicílico) o a cualquiera de los demás componentes (incluidos en la sección 6).
- Si después de tomar antiinflamatorios no esteroideos (AINE) o aspirina (ácido acetilsalicílico) se le ha producido una reacción alérgica; p. ej., asma, sibilancias, picor, secreción nasal, erupciones cutáneas, hinchazón.
- Si padece o ha padecido alguna vez dos o más episodios de úlcera o sangrado de estómago o úlceras intestinales.
- Si ha padecido alguna vez colitis grave (inflamación del intestino).
- Si ha padecido alguna vez problemas de coagulación de la sangre o problemas de sangrado después de tomar AINEs.
- Si se encuentra en el último trimestre del embarazo.
- Si padece insuficiencia cardíaca, renal o hepática grave.
- Si es niño o adolescente menor de 18 años.
Advertencias y precauciones.
Consulte a su médico o farmacéutico antes de empezar a usar Strefen Spray:
- Si está tomando otro Antiinflamatorio No Esteroideo (AINE) o aspirina.
- Si padece amigdalitis (inflamación de las amígdalas) o piensa que puede padecer una infección bacteriana de garganta (ya que puede necesitar antibióticos).
- Si tiene una infección - vea el apartado «Infecciones» a continuación.
- Si es paciente de edad avanzada (ya que es más probable que le produzca efectos adversos).
- Si padece o ha padecido alguna vez asma o tiene alergias.
- Si sufre una enfermedad de la piel denominada lupus eritematoso sistémico o enfermedad mixta del tejido conjuntivo.
- Si tiene hipertensión (tensión arterial elevada).
- Si tiene antecedentes de enfermedad intestinal (colitis ulcerosa, enfermedad de Crohn).
- Si tiene problemas de corazón, riñón o hígado.
- Si ha padecido un ictus.
- Si se encuentra en los primeros 6 meses del embarazo o está en periodo de lactancia.
Infecciones
Los medicamentos antiinflamatorios no esteroideos (AINE) pueden ocultar signos de infecciones como la fiebre y el dolor. Esto puede retrasar el inicio de un tratamiento adecuado para la infección, lo que puede conducir a un mayor riesgo de complicaciones. Si toma este medicamento mientras tiene una infección y sus síntomas persisten o empeoran, consulte a su médico o farmacéutico sin demora.
Mientras está usando Strefen Spray
- Ante el primer signo de una reacción cutánea (erupción, exfoliación, ampollas) u otro signo de una reacción alérgica, deje de usar este medicamento y consulte a un médico inmediatamente.
- Comunique a su médico cualquier síntoma abdominal inusual que se le pueda producir (especialmente sangrado).
- Consulte a su médico si no mejora, empeora, o si aparecen nuevos síntomas.
- El uso de medicamentos que contienen flurbiprofeno puede estar asociado a un pequeño incremento del riesgo de sufrir un ataque al corazón o ictus. Cualquier riesgo es más probable a dosis altas y en tratamientos prolongados. No exceda la dosis recomendada ni la duración del tratamiento(Ver Sección 3).
Niños
Este medicamento no lo pueden usar los niños ni los adolescentes menores de 18 años.
Otros medicamentos y Strefen Spray
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar otros medicamentos, incluso los adquiridos sin receta. En particular:
- Otros Antiinflamatorios No Esteroideos (AINEs), incluidos los inhibidores selectivos de la ciclooxigenasa-2 para el dolor o la inflamación, ya que pueden aumentar el riesgo de hemorragia en el estómago o intestino
- Warfarina, aspirina (ácido acetilsalicílico) y otros medicamentos anticoagulantes
- Inhibidores de la ECA, antagonistas de la angiotensina II (medicamentos que reducen la presión arterial)
- Diuréticos (incluidos los diuréticos ahorradores de potasio)
- ISRS (inhibidores selectivos de la recaptación de serotonina), para el tratamiento de la depresión
- Glucósidos cardíacos (para problemas del corazón) tales como la digoxina
- Ciclosporina (para impedir el rechazo de órganos después de un trasplante)
- Corticosteroides (para reducir la inflamación)
- Litio (para las depresiones)
- Metotrexato (para la psoriasis, la artritis y el cáncer)
- Mifepristona (medicamento utilizado para el aborto): como los AINEs pueden reducir el efecto de la mifepristona, no deben utilizarse en los 8-12 días posteriores a la administración de la mifepristona
- Antidiabéticos orales
- Fenitoína (para el tratamiento de la epilepsia)
- Probenecid, sulfinpirazona (para la gota y la artritis)
- Antibióticos quinolonas (para las infecciones bacterianas) tales como ciprofloxacino, levofloxacino
- Tacrolimus (inmunosupresor usado después del trasplante de órganos)
- Zidovudina (para el VIH).
Toma de Strefen Spray con alimentos, bebidas y alcohol
Se debe evitar la toma de alcohol durante el tratamiento con este medicamento, ya que puede incrementar el riesgo de hemorragia en el estómago o intestino.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
- No tome este medicamento si se encuentra en el último trimestre del embarazo.
- Si se encuentra en el primer semestre del embarazo o se encuentra en periodo de lactancia, consulte a su médico antes de tomar este medicamento.
El flurbiprofeno pertenece a un grupo de medicamentos (AINEs) que pueden alterar la fertilidad en las mujeres. Este efecto es reversible cuando se deja de tomar el medicamento.
Conducción y uso de máquinas
Este medicamento no debería afectar a su capacidad para conducir o usar máquinas. Sin embargo, si ocurrieran reacciones adversas como mareo y/o alteraciones visuales, no conduzca o utilice máquinas.
Información importante sobre algunos ingredientes de Strefen Spray:
Strefen Spray contiene parahidroxibenzoato de metilo (E218) y parahidroxibenzoato de propilo (E216)por lo que puede provocar reacciones alérgicas (posiblemente retardadas).
Este medicamento contiene menos de 23 mg de sodio (1mmol) por dosis; esto es, esencialmente “exento de sodio”.
Este medicamento contiene fragancias con alcohol anisílico, citral, citronelol, d-limoneno, geraniol y linalol.
Alcohol anisílico, citral, citronelol, d-limoneno, geraniol y linalol puede provocar reacciones alérgicas.
3. Cómo usar Strefen Spray
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, consulte a su médico o farmacéutico.
La dosis recomendadaes:
Adultos a partir de 18 años:
Aplicar 3 pulsaciones en la parte posterior de la garganta cada 3 - 6 horas según necesidad, hasta un máximo de 5 aplicaciones (15 pulsaciones) en 24 horas.
1 dosis (3 pulsaciones) contiene 8,75 mg de flurbiprofeno.
No utilice este medicamento en niños o adolescentes menores de 18 años.
Sólo para pulverización bucal
- Realice la pulverización únicamente en la parte posterior de la garganta.
- No inhale mientras realiza la pulsación.
- No aplique más de 5 aplicaciones (15 pulsaciones) cada 24 horas.
Strefen Sprayes sólo parauso a corto plazo.
Se debe usar la dosis efectiva más baja durante el menor tiempo necesario para aliviar los síntomas. Si tiene una infección, consulte a un médico o farmacéutico sin demora si los síntomas (como fiebre y dolor) persisten o empeoran (ver sección 2). Si aparece irritación en la boca, debe suspenderse el tratamiento con flurbiprofeno.
No utilice este medicamento durante más de 3 días, a menos que se lo recete su médico.
Si no mejora, empeora o si aparecen nuevos síntomas, consulte a su médico o farmacéutico.
Preparación (cebado) de la bomba
Tiene que preparar (cebar) la bomba antes de utilizarla por primera vez (o después de guardarla durante un periodo prolongado).
Apunte la boquilla hacia otra dirección (lejos de usted) y realice un mínimo de 4 pulsaciones hasta que aparezca una pulverización fina y homogénea. La bomba está lista para su uso (cebada). Si no ha utilizado el medicamento durante un periodo de tiempo prolongado, apunte la boquilla hacia otra dirección (lejos de usted) y realice un mínimo de 1 pulverización, asegurándose de que aparece una pulverización fina y homogénea. Asegúrese siempre de que aparece una pulverización fina y homogénea antes de usar el medicamento.
Uso del spray
Colocar el frasco en posición vertical con la boquilla dirigida hacia la parte posterior de la garganta.
Correcto Incorrecto
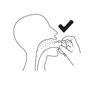
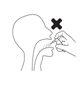
Aprete la bomba 3 veces, con un movimiento rápido y suave, asegurándose de apretarla a fondo en cada pulsación. Retire el dedo de la parte superior de la bomba entre cada pulsación.

No inhale mientras realiza la pulsación.
Si usa más Strefen Spray del que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a un médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Los síntomas de sobredosis pueden ser: náuseas o vómitos, dolor de estómago o, más raramente, diarrea. También puede aparecer ruido en los oídos, cefalea y sangrado gastrointestinal.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
DEJE DE TOMAR este medicamento y consulte a un médico inmediatamente si nota:
- Formas graves de una reacción cutánea tales como ampollas, incluyendo síndrome de Stevens-Johnson y necrólisis epidérmica tóxica (enfermedades médicas raras que se deben a reacciones adversas graves a medicacamentos o infecciones en las que se produce una reacción grave de la piel y la mucosa). Frecuencia: Desconocida (no pueden estimarse a partir de los datos disponibles).
- Signos de shock anafiláctico caracterizados por hinchazón de la cara, lengua o garganta que causa dificultad para respirar, palpitaciones, descenso de la presión arterial que causa shock (todos los efectos pueden aparecer incluso cuando se utiliza el medicamento por primera vez). Frecuencia: Rara (puede afectar hasta 1 de cada 1.000 personas).
- Signos de hipersensibilidad y reacciones cutáneas tales como enrojecimiento, hinchazón, exfoliación, ampollas, descamación o ulceración de la piel y la mucosa. Frecuencia: Poco frecuentes (puede afectar hasta 1 de cada 100 personas).
- Signos de una reacción alérgica como asma, sibilancias inexplicables o falta de aliento, picor, secreción nasal o erupciones cutáneas. Frecuencia: poco común (puede afectar hasta 1 de cada 100 personas).
Informe a su médico o farmacéutico si observa cualquiera de los siguientes efectos o cualquier efecto no descrito en este prospecto:
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Mareo, cefalea.
- Irritación de garganta.
- Úlceras de boca, dolor o entumecimiento en la boca.
- Dolor de garganta.
- Molestias en la boca (sensación de calor o quemazón u hormigueo).
- Náuseas y diarrea.
- Sensación de picor y prurito en la piel.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Adormecimiento.
- Ampollas en la boca o la garganta, entumecimiento en la garganta.
- Distensión abdominal, dolor abdominal, gases, estreñimiento, indigestión, vómitos.
- Boca seca.
- Sensación de quemazón en la boca, sentido del gusto alterado.
- Fiebre, dolor.
- Somnolencia o dificultad para conciliar el sueño.
- Empeoramiento del asma, sibilancias, falta de aliento.
- Reducción de la sensibilidad en la garganta.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- Anemia, trombocitopenia (bajo recuento de plaquetas en la sangre que puede dar lugar a moretones y sangrado).
- Hinchazón (edema), presión arterial alta, insuficiencia cardíaca o ataque al corazón.
- Hepatitis (inflamación del hígado).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.
También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano (www.notificaram.es). Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Strefen Spray
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
No refrigerar o congelar.
No utilice este medicamento durante más de 6 meses después de su primer uso.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE  de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Strefen Spray
El principio activo es flurbiprofeno. Una dosis (3 pulsaciones) contiene 8,75 mg de flurbiprofeno, equivalentes a 16,2 mg/ml de flurbiprofeno.
Los demás componentes (excipientes) son: Betadex, fosfato disódico dodecahidrato, ácido cítrico monohidrato, parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216), hidróxido de sodio, aroma de miel (contiene sustancia(s) aromatizante(s), preparado(s) aromatizante(s) y propilenglicol (E1520)), aroma de limón (contiene sustancia(s) aromatizante(s), preparado(s) aromatizante(s) y propilenglicol (E1520)), N,2,3-trimetil-2-isopropil butanamida, sacarina sódica, hidroxipropilbetadex y agua purificada.
Aspecto de Strefen Spray y contenido del envase
Strefen Spray es una solución transparente incolora o ligeramente amarilla con sabor a miel y limón.
Strefen Spray se presenta en un frasco blanco opaco de polietileno de alta densidad (HDPE) provisto de una bomba pulverizadora multicomponente con tapón protector de polipropileno.
Cada envase contiene 15 ml de solución que proporciona aproximadamente 83 pulsaciones.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Reckitt Benckiser Healthcare, S.A.
c/ Mataró, 28
08403 Granollers
España
Responsable de la fabricación
RB NL Brands B.V., Schiphol Blvd 207, 1118 BH Schiphol,
Países Bajos
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientesnombres
Reino Unido | Strefen 8.75mg/dose oromucosal spray |
Italia | Benactivdol gola antinfiammatorio |
Polonia | Strepsils Intensive o smaku wisni i miety |
Alemania | Dobendan Direkt Flurbiprofen Spray Honig- und Zitronengeschmack |
República Checa | Flurbiprofen Reckitt Benckiser |
Eslovaquia | Flurbiprofén Reckitt Benckiser 8,75 mg/ dávka orálna roztoková aerodisperzia |
Austria | Strepsils Spray Honig & Zitrone |
Bélgica | Strepfen spray met kers- en muntsmaak 8,75 mg/dose spray voor oromucosaal gebruik, oplossing |
Luxemburgo | Strepfen Spray Miel & Citron 8,75 mg/dose solution pour pulvérisation buccale |
Países Bajos | Strepfen Kers- en Munt 8,75 mg keelspray |
Hungría | Strepfen DIREKT méz és citrom ízu 16,2 mg/ml szájnyálkahártyán alkalmazott oldatos spray |
Rumanía | Strepsils Intensiv cirese si menta 8,75 mg/doza spray bucofaringian, solu?ie |
Bulgaria | ????????? ??? & ????? 8,75 mg/???? ????? ?? ????? ????????, ??????? |
Irlanda | Strepsils Intensive Cherry & Menthol 8.75 mg/dose Oromucosal Spray. |
España | Strefen Spray 8,75mg/dosis solución para pulverización bucal sabor miel y limón |
Portugal | Strepfen Spray Mel e Limão |
Croacia | Strepfen za odrasle s okusom meda i limuna 8,75 mg po dozi, sprej za usnu sluznicu, otopina |
Chipre | Strepfen Direct Honey & Lemon |
Dinamarca | Strefzap kirsebær og mint |
Estonia | Strepsils Intensive Sakura |
Finlandia | Strefen Hunaja & Sitruuna, Honung & Citron 16,2 mg/ml sumute suuonteloon, liuos |
Grecia | Streflam |
Islandia | Strefen Honung & Citron 16,2 mg/ml munnholsúði, lausn |
Letonia | Strepsils Intensive Cherry and Mint 16.2 mg/ml aerosols izsmidzinašanai mutes dobuma, škidums |
Lituania | Stepfen Direct Honey and Lemon |
Noruega | Strefen |
Eslovenia | Flurbiprofen Reckitt Benckiser 8,75 mg/odmerek oralno pršilo, raztopina |
Suecia | Strefen Körsbär & Mint |
Fecha de la última revisión de este prospecto: febrero2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es)
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a STREFEN SPRAY 8,75 MG/DOSIS SOLUCION PARA PULVERIZACION BUCAL SABOR MIEL Y LIMONForma farmacéutica: COMPRIMIDO BUCAL/PARA CHUPAR, 8,75 mgPrincipio activo: FlurbiprofenoFabricante: Alfasigma España, S.L.No requiere recetaForma farmacéutica: COMPRIMIDO BUCAL/PARA CHUPAR, 8,75 mgPrincipio activo: FlurbiprofenoFabricante: Alfasigma España, S.L.No requiere recetaForma farmacéutica: COMPRIMIDO BUCAL/PARA CHUPAR, 8,75 mgPrincipio activo: FlurbiprofenoFabricante: Laboratorios Cinfa S.A.No requiere receta
Médicos online para STREFEN SPRAY 8,75 MG/DOSIS SOLUCION PARA PULVERIZACION BUCAL SABOR MIEL Y LIMON
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de STREFEN SPRAY 8,75 MG/DOSIS SOLUCION PARA PULVERIZACION BUCAL SABOR MIEL Y LIMON, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








