STRATTERA 4 MG/ML SOLUCION ORAL

Cómo usar STRATTERA 4 MG/ML SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Strattera 4mg/ml solución oral
atomoxetina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Strattera y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Strattera
- Cómo tomar Strattera
- Posibles efectos adversos
- Conservación de Strattera
- Contenido del envase e información adicional
1. Qué es Strattera y para qué se utiliza
Para qué se utilizaStrattera contiene atomoxetina y se utiliza en el tratamiento del trastorno por déficit de atención con hiperactividad (TDAH). Se utiliza
- en niños a partir de los 6 años
- en jóvenes
- en adultos
Se utiliza únicamente como parte del tratamiento completo de la enfermedad que requiere también tratamientos que no incluyen medicamentos, como asesoramiento o terapia de conducta.
No se utiliza para el tratamiento del TDAH en niños menores de 6 años debido a que no se conoce si el fármaco funciona o si es seguro en esta población.
En adultos, Strattera se usa para el tratamiento del TDAH cuando los síntomas son muy problemáticos y afecta a su trabajo o vida social y cuando ha tenido síntomas de la enfermedad cuando era niño.
Cómo funcionaStrattera incrementa la cantidad de noradrenalina en el cerebro. Ésta es una sustancia química producida de forma natural y que incrementa la atención y disminuye la impulsividad e hiperactividad en los pacientes con TDAH. Este medicamento le ha sido prescrito para ayudar a controlar los síntomas del TDAH. Este medicamento no es un estimulante y por lo tanto no provoca adicción.Pueden tardar algunas semanas desde que comience el tratamiento con el medicamento hasta que se mejoren por completo los síntomas.
Sobre el TDAHNiños y jóvenes con TDAH encuentran:
- dificultad para estar sentados
- dificultad para concentrarse
No es culpa suya que no puedan hacer estas cosas. A muchos niños y jóvenes esto les resulta difícil. No obstante a los pacientes con TDAH esto puede causarles problemas en el día a día. Niños y jóvenes con TDAH pueden tener dificultad al aprender y hacer los deberes. Tienen dificultad para comportarse bien en casa, en el colegio o en otros lugares. El TDAH no afecta a la inteligencia de un niño o de un joven.
Adultos con TDAH encuentran difícil hacer todas las cosas que los niños también encuentran difícil, sin embargo esto puede suponer que tengan problemas con:
- trabajo
- relaciones
- baja autoestima
- educación
2. Qué necesita saber antes de empezar a tomar Strattera
NO tome Strattera si usted
- es alérgico a la atomoxetina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- ha tomado en las últimas dos semanas un medicamento de los denominados inhibidores de la monoaminoxidasa (IMAO), por ejemplo fenelzina. Los IMAO se utilizan a veces para la depresión y otros trastornos mentales; tomar Strattera junto con un IMAO podría causar efectos adversos graves o constituir una amenaza para la vida. Asimismo debe esperar al menos 14 días después de terminar su tratamiento con Strattera antes de tomar un IMAO.
- tiene una enfermedad ocular llamada glaucoma de ángulo estrecho (aumento de la presión en los ojos).
- tiene problemas graves de corazón que pueden empeorar por un incremento en la frecuencia cardíaca y/o en la presión sanguínea, lo cual puede ocurrir con Strattera.
- tiene problemas graves en los vasos sanguíneos de su cerebro, tales como un derrame cerebral, parte de un vaso sanguíneo inflamado y debilitado (aneurisma) o vasos sanguíneos estrechos u obstruidos.
- tiene un tumor de su glándula adrenal (feocromocitoma).
No tome Strattera si le aplica alguno de los casos anteriores. Si no está seguro, consulte a su médico o farmacéutico antes de tomar Strattera debido a que Strattera puede empeorar estos problemas.
Advertencias y precauciones
Tanto los adultos como los niños deben tener en cuenta las siguientes advertencias y precauciones. Consulte a su médico o farmacéutico antes de empezar a tomar Strattera si tiene:
- ideas o intento de suicidio.
- problemas cardíacos (incluyendo defectos cardíacos) o una frecuencia cardíaca aumentada. Strattera puede aumentar su frecuencia cardíaca (pulso). Se han notificado casos de muerte súbita en pacientes con defectos cardíacos.
- la presión sanguínea elevada. Strattera puede aumentar su presión sanguínea.
- la presión sanguínea baja. Strattera puede causar mareos o desvanecimientos en personas que tengan la presión sanguínea baja.
- problemas con cambios repentinos en su presión sanguínea o frecuencia cardíaca.
- enfermedad cardiovascular o antecedentes de haber sufrido un accidente cerebrovascular.
- problemas de hígado. Puede necesitar una dosis menor.
- reacciones psicóticas incluyendo alucinaciones (escuchar voces o ver cosas irreales), creer cosas que no son ciertas o mostrarse desconfiado.
- manía (sentirse exaltado o sobreexcitado, lo cual provoca un comportamiento inusual) y agitación.
- sentimiento agresivo.
- sentimiento de antipatía y enfado (hostilidad).
- antecedentes de epilepsia o ha padecido convulsiones por alguna otra razón. Strattera podría producirle un aumento en la frecuencia de las convulsiones.
- estado de ánimo diferente al habitual (cambios de humor) o sentimiento de infelicidad.
- espasmos repetidos de difícil control de cualquier parte del cuerpo o repetición de sonidos y palabras.
Consulte con su médico o farmacéutico si tiene cualquiera de los síntomas mencionados anteriormente antes de comenzar el tratamiento, debido a que Strattera puede hacer que estos problemas empeoren. Su médico querrá hacer un seguimiento sobre cómo el medicamento le afecta.
El tratamiento con Strattera puede hacerle sentir agresivo, hostil o violento; o agravar estos síntomas si estaban presentes antes del tratamiento. También puede causarle cambios inusuales en su comportamiento o estado de ánimo (incluyendo agresión física, comportamiento amenazante y pensamientos de dañar a otros). Si usted o su familia y/o amigos notan alguna de estas reacciones, hable con su médico o farmacéutico de inmediato.
Síndrome serotoninérgico
El síndrome serotoninérgico es una situación que amenaza potencialmente la vida que puede aparecer cuando se toma Strattera en combinación con otros medicamentos (ver sección 2 “Otros medicamentos y Strattera”). Los signos y síntomas del síndrome serotoninérgico pueden incluir una combinación de los siguientes: confusión, inquietud, falta de coordinación y rigidez, alucinaciones, coma, latidos cardíacos rápidos, aumento de la temperatura corporal, cambios rápidos en la presión arterial, sudoración, enrojecimiento, temblores, reflejos hiperactivos, náuseas, vómitos y diarrea. Contacte con un médico o vaya inmediatamente al servicio de urgencias del hospital más cercano si cree que le está sucediendo el síndrome serotoninérgico.
Pruebas que su médico realizará antes de que empiece a tomar Strattera
Estas pruebas son para decidir si Strattera es el medicamento correcto para usted.
Su médico medirá su
- presión sanguínea y su frecuencia cardiaca (pulso) antes y durante el tiempo que usted esté tomando Strattera
- peso y su altura si durante el tiempo en el que esté tomando Strattera, usted es un niño o un adolescente
Su médico le consultará sobre:
- otros medicamentos que usted esté tomando
- si tiene antecedentes familiares de muerte súbita
- cualquier otro problema médico (tales como problemas cardíacos) que usted o su familia puedan tener.
Es importante que proporcione toda la información que pueda. Esto ayudará a su médico a decidir si Strattera es el medicamento correcto para usted. Su médico puede decidir hacer otras pruebas médicas antes de empezar el tratamiento con este medicamento.
Otros medicamentos y Strattera
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto incluye los adquiridos sin receta médica. Su médico decidirá si puede tomar Strattera con otros medicamentos y en algunos casos, puede que su médico necesite ajustar la dosis o aumentarla más lentamente.
No tome Strattera con los medicamentos llamados IMAOs (inhibidores de la monoaminoxidasa) tomados para la depresión. Ver sección 2 “No tome Strattera”.
Si está tomando otros medicamentos, Strattera puede afectar al funcionamiento correcto de estos o provocar efectos adversos. Si usted está tomando cualquier de los siguientes medicamentos, compruebe con su médico o farmacéutico antes de tomar Strattera:
- medicamentos que incrementan la presión sanguínea o son utilizados para controlarla.
- medicamentos tales como antidepresivos, por ejemplo imipramina, venlafaxina, mirtazapina, fluoxetina y paroxetina.
- algunos remedios para la tos y el resfriado que contengan medicinas que pueden afectar a la presión sanguínea. Cuando compre este tipo de productos, es importante comprobarlos con su farmacéutico.
- algunos medicamentos utilizados para tratar trastornos mentales.
- medicamentos que se sabe que aumentan el riesgo de convulsiones.
- algunos medicamentos que hacen que Strattera permanezca en el cuerpo más tiempo de lo normal (tales como quinidina y terbinafina).
- salbutamol (un medicamento para el tratamiento del asma) cuando se toma por vía oral o se inyecta, puede tener la sensación de que su corazón se acelera, pero esto no empeorará su asma.
Los medicamentos descritos debajo pueden aumentar el riesgo de presentar un ritmo cardíaco anormal cuando se está tomando Strattera:
- medicamentos utilizados para controlar el ritmo cardíaco,
- medicamentos que alteran el contenido de sales de la sangre,
- medicamentos para la prevención y el tratamiento de la malaria,
- antibióticos (como eritromicina y moxifloxacino).
Si no está seguro sobre si los medicamentos que está tomando están en la lista de medicamentos comentada antes, consulte con su médico o farmacéutico antes de tomar Strattera.
Strattera puede afectar o verse afectado por otros medicamentos. Estos incluyen:
- Algunos antidepresivos, opioides como tramadol y medicamentos utilizados para tratar la migraña llamados triptanes. Estos medicamentos pueden interactuar con Strattera y provocar el síndrome serotoninérgico, una situación que amenaza potencialmente la vida. (Ver sección 2, Advertencias y precauciones, Síndrome serotoninérgico).
Embarazo y lactancia
No se sabe si este medicamento puede afectar al feto o puede pasar a la leche materna.
- Este medicamento no debería tomarse durante el embarazo a no ser que su médico le indique lo contrario.
- Debería evitar tomar este medicamento si está dando el pecho o dejar de dar el pecho.
Si usted:
- está embarazada o en periodo de lactancia,
- cree que podría estar embarazada o tiene intención de quedarse embarazada,
- tiene intención de dar el pecho,
consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Strattera puede producirle cansancio, somnolencia o mareos. Tenga cuidado si conduce o utiliza maquinaria hasta que sepa cómo le afecta la toma de Strattera. Si se siente cansado, con somnolencia o mareado, no debería conducir o utilizar maquinaria.
Información importante sobre la solución oral
Esta solución oral le puede irritar los ojos. Si la solución oral entra en contacto con los ojos, enjuágueselos inmediatamente con abundante agua y consulte a su médico. Si las manos o cualquier otra parte del cuerpo entran en contacto con la solución oral, también deberá enjuagarse con agua lo más rápidamente posible.
Strattera solución oral contiene sorbitol, sodio, benzoato de sodio y propilenglicol
Este medicamento contiene 32,97 mg de sorbitol en cada ml. El sorbitol es una fuente de fructosa. Si su médico le ha indicado que usted (o su hijo) padecen una intolerancia a ciertos azúcares, o se les ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte usted (o su hijo) con su médico antes de tomar este medicamento.
Este medicamento contiene 2,64 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada ml. Esto equivale al 3,3 % de la ingesta diaria máxima de sodio recomendada para un adulto.
Este medicamento contiene 0,8 mg de benzoato de sodio en cada ml.
Este medicamento contiene 9,8 mg de propilenglicol en cada ml.
3. Cómo tomar Strattera
?
- Los niños no deben tomar este medicamento sin la ayuda de un adulto.
- Si siente somnolencia o náuseas cuando está tomando Strattera una vez al día, su médico puede cambiar la toma de medicación a dos veces al día.
- El medicamento se puede tomar con o sin alimentos.
- La solución oral no se debe mezclar con comida o agua ya que puede disminuir la cantidad ingerida o hacer que el sabor sea menos agradable.
- El tomar el medicamento siempre a la misma hora cada día puede ayudar a recordarle que debe tomarlo.
Strattera solución oral está disponible en un frasco. Esto forma parte de un envase que también incluye un dispositivo de dosificación que se compone de una jeringa para dosificación oral de 10 ml graduada en fracciones de 1 ml y un adaptador a presión del frasco. Para ver las instrucciones sobre cómo usar el adaptador y la jeringa dosificadora, lea las instrucciones del manual del usuario que se incluye en el envase.
Cuánto tomarSi es un niño (a partir de 6 años) o un adolescente:Su médico le indicará la dosis de Strattera que debe tomar en función de su peso. Normalmente deberá comenzar con una dosis baja antes de aumentarla, en función de su peso.
- Hasta 70 kg de peso: comenzando con una dosis diaria total de 0,5 mg por kg de peso durante un mínimo de 7 días. Su médico decidirá entonces si se aumenta a la dosis habitual de mantenimiento de 1,2 mg por kg de peso y por día.
- Más de 70 kg de peso: comenzando con una dosis diaria total de Strattera 40 mg durante un mínimo de 7 días. Su médico decidirá entonces si se aumenta a la dosis habitual de mantenimiento de 80 mg al día. La dosis máxima diaria es de 100 mg.
Adultos
- Se debería comenzar Strattera con una dosis diaria total de 40 mg durante un mínimo de 7 días. Su médico decidirá entonces si se aumenta a la dosis habitual de mantenimiento de 80 mg a 100 mg al día. La dosis máxima diaria es de 100 mg.
Si tiene problemas de hígado, su médico le puede recomendar una dosis menor.
Si toma más Strattera del que debeEn caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.Los síntomas comunicados más comúnmente tras una sobredosis son síntomas gastrointestinales, somnolencia, mareos, temblor y comportamiento anormal. Muy raramente, también se ha notificado el síndrome serotoninérgico, una situación que amenaza potencialmente la vida. (Ver sección 2, Advertencias y precauciones, Síndrome serotoninérgico).
Si olvidó tomar StratteraSi olvida una dosis, tome otra tan pronto como sea posible, pero no tome una cantidad que sobrepase la dosis diaria total en un periodo de 24 horas. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con StratteraSi deja de tomar Strattera, normalmente no aparecen efectos adversos pero los síntomas del TDAH pueden reaparecer. Debería hablar con su médico antes de interrumpir el tratamiento.
Lo que hará su médico cuando esté en tratamiento
Su médico le hará algunas pruebas
- antes de empezar - se asegurará que Strattera es seguro y le beneficiará.
- después de empezar - las pruebas se harán al menos cada 6 meses, aunque posiblemente serán más frecuentes.
Las pruebas también se realizarán cuando se modifique la dosis. Estas pruebas incluirán:
- medición de la altura y el peso en niños y jóvenes
- medición de la presión sanguínea y la frecuencia cardíaca
- comprobar si tiene algún problema o si los efectos adversos han empeorado mientras está tomando Strattera
Tratamiento a largo plazoStrattera no necesita tomarse indefinidamente. Si toma Strattera durante más de un año, su médico debe revisar su tratamiento para comprobar si el medicamento es aún necesario.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Aunque algunas personas sufren efectos adversos, la mayoría considera que Strattera les ayuda. Su médico le hablará sobre estos efectos adversos.
Algunos efectos adversos podrían ser graves. Si presenta alguno de los efectos descritos a continuación, póngase inmediatamente en contacto con su médico.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- sentir o tener un ritmo cardíaco muy rápido, ritmo cardíaco anormal
- pensamiento o sentimiento de suicidio
- sentimiento agresivo
- sentimiento antipatía y de enfado (hostilidad)
- cambios de humor
- reacción alérgica grave con síntomas de
- hinchazón de la cara y garganta
- dificultad para respirar
- urticaria (pequeñas ronchas rojizas y con picor en la piel)
- convulsiones
- síntomas psicóticos incluyendo alucinaciones (tales como escuchar voces o ver cosas que no existen), creer cosas que no son ciertas o mostrarse desconfiado.
Niños y jóvenes menores de 18 años tienen mayor riesgo de sufrir efectos adversos tales como:
- pensamiento o sentimiento de suicidio (pueden afectar hasta 1 de cada 100 personas)
- cambios de humor (pueden afectar hasta 1 de cada 10 personas)
Los adultos tienen un riesgo menor(pueden afectar hasta 1 de cada 1.000 personas) de sufrir efectos adversos tales como:
- convulsiones
- síntomas psicóticos incluyendo alucinaciones (tales como escuchar voces o ver cosas que no existen), creer cosas que no son ciertas o mostrarse desconfiado
Raras(pueden afectar hasta 1 de cada 1.000 personas)
- afectación del hígado
Interrumpa el tratamiento con Strattera y póngase inmediatamente en contacto con su médico si aparece alguno de los efectos adversos siguientes:
- orina oscura
- piel y ojos amarillentos
- dolor al palparse en la parte superior derecha del abdomen, justo debajo de las costillas
- sensación de malestar (náuseas) sin explicación
- cansancio
- picor
- sensación de empezar con un resfriado
Otros efectos adversos notificados han sido los siguientes. Si alguno de ellos empeora consulte con su médico o farmacéutico.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas) | |
NIÑOS mayores de 6 años y JÓVENES | ADULTOS |
Estos efectos pueden desaparecer pasado un tiempo en la mayoría de los pacientes |
|
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas) | |
NIÑOS mayores de 6 años y JÓVENES | ADULTOS |
|
|
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas) | |
NIÑOS mayores de 6 años y JÓVENES | ADULTOS |
|
|
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas) | |
NIÑOS mayores de 6 años y JÓVENES | ADULTOS |
|
|
Frecuencia no conocida | |
NIÑOS mayores de 6 años y JÓVENES | ADULTOS |
|
Efectos sobre el crecimientoCuando algunos niños comienzan a tomar Strattera, su crecimiento (peso y altura) se ve disminuido. Sin embargo, con un tratamiento a largo plazo, los niños recuperan el peso y la altura adecuada a su rango de edad. Su médico vigilará la altura y el peso de su hijo. Si su hijo no crece o gana peso como se espera, su médico puede cambiar la dosis de Strattera o suspender temporalmente el tratamiento con Strattera.
Comunicación de efectos adversosSi experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Strattera
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el frasco después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No utilice la solución oral más de 45 días después de la primera apertura del frasco.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Strattera 4mg/ml
- El principio activo es hidrocloruro de atomoxetina. Cada ml de solución oral contiene hidrocloruro de atomoxetina en cantidad equivalente a 4 mg de atomoxetina.
- Los demás componentes son benzoato de sodio (E211), dihidrogenofosfato de sodio dihidrato, ácido fosfórico, sorbitol líquido (cristalizable) E420, xilitol, sabor artificial de frambuesa (que contiene propilenglicol E1520), sucralosa, hidróxido de sodio, agua purificada.
Aspecto del producto y contenido del envaseSolución oral, 4 mg/ml (transparente incolora)
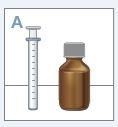
Strattera solución oral está disponible en un frasco que contiene 100 ml de solución, con tapón de seguridad para niños. El envase también incluye un dispositivo de dosificación que se compone de una jeringa para dosificación oral de 10 ml graduada en fracciones de 1 ml y un adaptador a presión del frasco.
Strattera solución oral está disponible en un envase que contiene un frasco individual y en un envase múltiple con tres frascos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricaciónTitular de la autorización de comercialización:Lilly S.A. Avda de la Industria 30, 28108 Alcobendas, Madrid, España.
Responsable de fabricación:Patheon France, 40 Boulevard de Champaret, 38300 Bourgoin-Jallieu, Francia.
Strattera es una marca registrada de Eli Lilly and Company Limited.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:Irisfarma S.A. Avda. de la Industria 30, 28108 Alcobendas, Madrid, España.
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:Austria, Dinamarca, Alemania, Hungría, , Irlanda, Italia, Malta, Noruega, Portugal, Rumanía, Eslovenia, España, Suecia : Strattera.
Fecha de la última revisión de este prospecto: Noviembre 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Stratteraatomoxetinasolución oral
Instrucciones de UsoSu guía paso a paso para usar STRATTERACUANDO USE STRATTERA, lea y siga cuidadosamente las instrucciones paso a paso.
ADVERTENCIA:El adaptador supone un RIESGO DE ASFIXIA por ser un componente pequeño. No una la jeringa al adaptador hasta que el adaptador esté completamente insertado en el frasco. Para un uso seguro, el adaptador se debe insertar completamente en el frasco. Utilizar solo bajo supervisión de un adulto.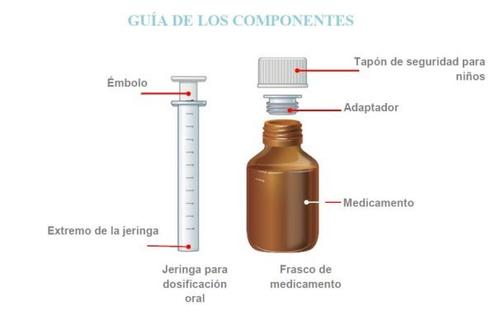 .IMPORTANTENOdeje que su hijo tome el medicamento sin su ayuda.NOuse el medicamento si ha caducado (comprobar la fecha de caducidad en la etiqueta).NOuse el medicamento si han pasado más de 45 días desde la primera vez que abrió el frasco. Ver la sección de Eliminaciónpara conocer más sobre qué hacer con la medicación que ya no usa.NOlave la jeringa para dosificación oral con jabón o detergente. NOponga la jeringa en el lavavajillas. Si lo hace puede que la jeringa no funcione tan bien como debería. Por favor, ver las instrucciones de limpieza desde el paso N al P.No se recomienda mezclar STRATTERA solución oral con comida o agua debido a que puede afectar al sabor o impedir que se tome una dosis completa.STRATTERA provoca irritación en los ojos. Evite el contacto con los ojos.Si el medicamento entra en contacto con los ojos, lávelos inmediatamente con agua y consulte a su médico. Lave las manos y las superficies que hayan podido entrar en contacto con el medicamento tan pronto como sea posible.
.IMPORTANTENOdeje que su hijo tome el medicamento sin su ayuda.NOuse el medicamento si ha caducado (comprobar la fecha de caducidad en la etiqueta).NOuse el medicamento si han pasado más de 45 días desde la primera vez que abrió el frasco. Ver la sección de Eliminaciónpara conocer más sobre qué hacer con la medicación que ya no usa.NOlave la jeringa para dosificación oral con jabón o detergente. NOponga la jeringa en el lavavajillas. Si lo hace puede que la jeringa no funcione tan bien como debería. Por favor, ver las instrucciones de limpieza desde el paso N al P.No se recomienda mezclar STRATTERA solución oral con comida o agua debido a que puede afectar al sabor o impedir que se tome una dosis completa.STRATTERA provoca irritación en los ojos. Evite el contacto con los ojos.Si el medicamento entra en contacto con los ojos, lávelos inmediatamente con agua y consulte a su médico. Lave las manos y las superficies que hayan podido entrar en contacto con el medicamento tan pronto como sea posible.
PASO 1 DISPOSICIÓN INICIAL DEL FRASCO
| Solo antes del primer uso, empuje el adaptador hasta que se ajuste completamenteal frasco. No ensamble la jeringa al adaptador hasta que el adaptador esté completamente insertado en el frasco.NOgire el adaptador.ADVERTENCIA:El adaptador supone un RIESGO DE ASFIXIA por ser un componente pequeño. Para un uso seguro, se debe insertar completamente en el frasco. |
PASO 2 PREPÁRESE
| Reúna todos los elementos que necesitará:
|
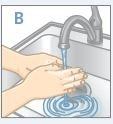
| Lávese las manos con agua y jabón |
Escriba la dosis de su hijo aquí:______ mlAsegúrese de que es la dosis exacta que el médico le prescribió.Si la dosis de su hijo es10 ml O MENOS,usará la jeringa 1 vezSi la dosis de su hijo esMÁS DE 10 mlpero NO más de 20 ml, usará la misma jeringa 2 veces.Si la dosis de su hijo esMÁS DE 20 ml,usará la misma jeringa 3 veces.PASO 3 OBTENCIÓN DE LA DOSIS
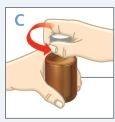
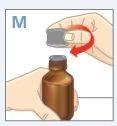
| Apriete el tapón de rosca hacia abajo mientras lo gira en el sentido contrario a las agujas del reloj.Retire el tapón de rosca del frasco. |
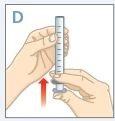
| Empuje el émbolo completamente hasta el extremode la jeringa. |
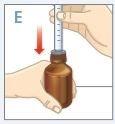
| Inserte el extremo de la jeringa en el adaptador.Asegúrese de que el extremo de la jeringa está completamente ajustado enel adaptador. |
| Gire hacia abajo el frasco y la jeringa. |
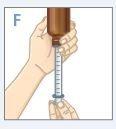
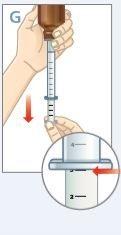
| Mantenga el frasco y la jeringa con 1 mano. Con la otra mano, tire hacia abajo del émbolo para extraer la cantidad apropiada de medicamento en la jeringa.Compruebe que la dosis en la jeringa es la apropiada. |
| Gire el frasco hacia arriba y colóquelo en una superficie plana.Compruebe que no haya ninguna burbuja de aire en la jeringa. Si la hay, vuelva a introducir el medicamento en el frasco y repita los pasos del D hasta el H. Unaburbuja de aire puede resultar en una dosis incorrecta. |
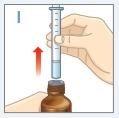
| Retire la jeringa del frasco. NOtoque el émbolo. |
PASO 4 ADMINISTRACIÓN DEL MEDICAMENTO
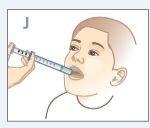
| Coloque el extremo de la jeringa en un lado de la boca de su hijo. Diga a su hijo que no muerda la jeringa. |
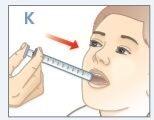
| Empuje el émbolo hacia abajo hasta que TODO el medicamento esté en la boca de su hijo.NOdirija directamente la medicación hacia la garganta de su hijo.Asegúrese de que su hijo traga todo el medicamento. |
| Si la dosis de su hijo esMAYOR DE 10 ml, repita desde el paso D al K para darle la dosis restante. Utilice la misma jeringa. Ver el cuadro de dosificacionesen la otra cara de este documento.Asegúrese de que administra la dosis exacta que el médico de su hijo le prescribió. |
PASO5LIMPIEZACARA 1
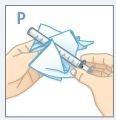
| Vuelva a enroscar el tapón del frasco firmemente. NOretire el adaptador. El tapón se adaptará. |
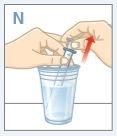
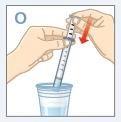
| Llene un vaso con agua limpia.NOlave la jeringa para dosificación con jabón o detergente.NOretire el émbolo de la jeringa para dosificación oral. Coloque el extremo de la jeringa en el agua. Tire del émbolo hacia arriba para llenarla con agua. |
| Empuje el émbolo hacia abajo y vacíe el agua en el vaso o en el lavabo. |
| Elimine el exceso de agua de la jeringa.Seque la jeringa con un pañuelo de papel. |
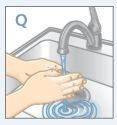
| Lávese las manos y las de su hijo con jabón y agua.NO se toquelos ojos después de estar en contacto con STRATTERA.STRATTERA puede irritar sus ojos. |
Ver la otra cara de este documento para obtener respuesta a las preguntas más frecuentes, comprobar el cuadrodedosificacióny otra información importante.
CARA 2
CUADRODEDOSIFICACIÓNPARADOSISDEMÁSDE10mlUse este cuadro para calcular la dosis a administrar a su hijo. Busque la dosis correcta en la primera columna.Consulte con su médico o farmacéutico como administrar la dosis correcta a su hijo.
Si esta es la dosis de su hijo | Ponga esta cantidad de STRATTERA en la jeringa, por primera vez | Ponga esta cantidad de STRATTERA en la jeringa, una segunda vez | Ponga esta cantidad de STRATTERA en la jeringa, una tercera vez |
11 ml | 10 ml | 1 ml | 0 |
12 ml | 10 ml | 2 ml | 0 |
13 ml | 10 ml | 3 ml | 0 |
14 ml | 10 ml | 4 ml | 0 |
15 ml | 10 ml | 5 ml | 0 |
16 ml | 10 ml | 6 ml | 0 |
17 ml | 10 ml | 7 ml | 0 |
18 ml | 10 ml | 8 ml | 0 |
19 ml | 10 ml | 9 ml | 0 |
20 ml | 10 ml | 10 ml | 0 |
21 ml | 10 ml | 10 ml | 1 ml |
22 ml | 10 ml | 10 ml | 2 ml |
23 ml | 10 ml | 10 ml | 3 ml |
24 ml | 10 ml | 10 ml | 4 ml |
25 ml | 10 ml | 10 ml | 5 ml |
Preguntas frecuentesP.¿Qué sucede si hay una burbuja de aire en la jeringa para dosificación oral?R.NOadministre el medicamento a su hijo. Las burbujas pueden hacer que la dosis sea incorrecta.Vacíe de nuevo el medicamento en el frasco y repita desde el paso D al H.P.¿Qué sucede si hay demasiado medicamento en la jeringa?R.Mantenga el extremo de la jeringa en el frasco. Sujete el frasco hacia arriba.Empuje hacia abajo el émbolo hasta que la dosis correcta esté en la jeringa. P.¿Qué sucede si no hay suficiente medicamento en la jeringa?R.Mantenga el extremo de la jeringa en el frasco. Sujete el frasco hacia abajo. Tirar delémbolo hacia abajo hasta que la dosis correcta esté en la jeringa.P.¿Qué hago si el medicamento entra en contacto con mis ojos o con los de mi hijo?R.Lave los ojos inmediatamente con agua y consulte a su médico. Tan pronto como seaposible, lávese las manos y la superficie que haya podido estar en contacto con el medicamento.P.¿Cómo viajo con este medicamento?R.Asegúrese que tiene suficiente STRATTERA para todo el viaje. Conserve el medicamento enun lugar seguro boca arriba a temperatura ambiente.P.¿Puedo mezclar STRATTERA con comida o agua antes de dárselo a mi hijo?R.No se recomienda mezclar STRATTERA solución oral con comida o con agua. Puede afectar al saborde la solución oral o evitar que se administre la dosis completa. Su hijo puedebeber un vaso de agua después de haber tomado la dosis entera de la medicación.ConservaciónEste medicamento no necesita condiciones especiales de conservación.Mantener el frasco y la jeringa fuera de la vista y del alcance de los niños.EliminaciónNo tire los medicamentos directamente al fregadero o a la basura.Consulte a su farmacéutico como tirar los medicamentos que ya no va a usar más.Estas medidas ayudarán a proteger el medioambiente.
- País de registro
- Precio medio en farmacia106.15 EUR
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a STRATTERA 4 MG/ML SOLUCION ORALForma farmacéutica: CAPSULA, 10 mgPrincipio activo: atomoxetineFabricante: Laboratorios Rubio S.A.Requiere recetaForma farmacéutica: CAPSULA, 100 mgPrincipio activo: atomoxetineFabricante: Laboratorios Rubio S.A.Requiere recetaForma farmacéutica: CAPSULA, 18 mgPrincipio activo: atomoxetineFabricante: Laboratorios Rubio S.A.Requiere receta
Médicos online para STRATTERA 4 MG/ML SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de STRATTERA 4 MG/ML SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes