STALEVO 75 mg/18,75 mg/200 mg COMPRIMIDOS RECUBIERTOS CON PELICULA

Cómo usar STALEVO 75 mg/18,75 mg/200 mg COMPRIMIDOS RECUBIERTOS CON PELICULA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Stalevo 75mg/18,75mg/200mg comprimidos recubiertos con película
Levodopa/carbidopa/entacapona
Lea todo el prospecto detenidamente antes de comenzar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Stalevo y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Stalevo
- Cómo tomar Stalevo
- Posibles efectos adversos
- Conservación de Stalevo
- Contenido del envase e información adicional
1. Qué es Stalevo y para qué se utiliza
Stalevo contiene tres principios activos (levodopa, carbidopa y entacapona) en un comprimido recubierto con película. Stalevo se utiliza para tratar la enfermedad de Parkinson.
La enfermedad de Parkinson se debe a la baja concentración en el cerebro de una sustancia llamada dopamina. La levodopa aumenta la cantidad de dopamina y, de este modo, reduce los síntomas de la enfermedad de Parkinson. Carbidopa y entacapona mejoran los efectos antiparkinsonianos de levodopa.
2. Qué necesita saber antes de empezar a tomar
No tome Stalevo si:
- es alérgico a levodopa, carbidopa o entacapona, o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- padece glaucoma de ángulo estrecho (un trastorno ocular)
- tiene un tumor de las glándulas suprarrenales
- toma determinados medicamentos para tratar la depresión (combinaciones de inhibidores selectivos de la MAO‑A y MAO‑B, o inhibidores no selectivos de la MAO)
- ha sufrido alguna vez síndrome neuroléptico maligno (SNM – una reacción rara a los medicamentos que se emplean en el tratamiento de trastornos mentales graves)
- ha sufrido alguna vez rabdomiolisis no traumática (un trastorno muscular raro)
- sufre un trastorno grave del hígado.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Stalevo si sufre o ha sufrido alguna vez:
- un ataque cardiaco o cualquier otra enfermedad del corazón, incluidas las arritmias, o de los vasos sanguíneos
- asma o cualquier otra enfermedad pulmonar
- un problema del hígado, porque puede ser necesario ajustar la dosis que recibe
- alguna enfermedad del riñón o relacionada con las hormonas
- úlcera de estómago o convulsiones
- diarrea prolongada. Consulte con su médico ya que puede ser un signo de inflamación del colon
- cualquier forma de trastorno mental grave, como psicosis
- glaucoma de ángulo abierto, porque puede que sea necesario ajustar la dosis y controlarle la presión intraocular.
Consulte a su médico si está tomando:
- antipsicóticos (medicamentos para tratar la psicosis)
- algún medicamento que pueda causar un descenso de la presión sanguínea al levantarse de una silla o de la cama. Debe saber que Stalevo puede empeorar estas reacciones.
Consulte a su médico si durante el tratamiento con Stalevo:
- nota gran rigidez en los músculos o que se contraen con violencia, o si sufre temblores, agitación, confusión, fiebre, pulso rápido o grandes fluctuaciones de la presión arterial. Si se produce alguna de estas circunstancias, póngase en contacto con su médico de inmediato
- se siente deprimido, tiene ideas de suicidio o nota alteraciones inusuales de su comportamiento
- observa que se queda dormido de repente, o se nota muy somnoliento. Si esto le sucede, no debe conducir ni manejar herramientas o maquinaria (ver también la sección “Conducción y uso de máquinas”)
- empieza a notar movimientos incontrolados o éstos empeoran. Si esto ocurre, su médico podría verse obligado a cambiarle la dosis de medicación
- sufre diarrea; se recomienda vigilar el peso para evitar una posible pérdida de peso excesiva
- sufre pérdida de apetito progresiva, astenia (debilidad, agotamiento) y descenso de peso en un tiempo relativamente breve. En estos casos, se debe valorar la posibilidad de una evaluación médica completa que incluya un examen de la función hepática
- considera que tiene que dejar de tomar Stalevo; consulte la sección "Si interrumpe el tratamiento con Stalevo".
Informe a su médico si usted, o su familia/cuidador, nota que está desarrollando síntomas similares a la adicción, que conducen a un deseo de grandes dosis de Stalevo y otros medicamentos utilizados para tratar la enfermedad de Parkinson.
Informe a su médico si usted o su familia/cuidador nota que está desarrollando deseos o propensión a comportarse de una manera inusual en usted o que no puede resistir el impulso, la determinación o la tentación de realizar ciertas actividades que pueden causarle daño a usted o a los demás. Estos comportamientos se denominan trastornos del control de impulsos y pueden incluir adicción al juego, comida o gastos excesivos, y un impulso sexual anormalmente elevado o preocupación con un aumento de pensamientos o sensaciones sexuales. Su médico puede necesitar revisar su tratamiento.
Durante el tratamiento prolongado con Stalevo, el médico puede hacerle pruebas analíticas con regularidad.
Si tiene que someterse a una intervención quirúrgica, debe informar a su médico de que está tomando Stalevo.
No se recomienda utilizar Stalevo para el tratamiento de los síntomas extrapiramidales (por ejemplo, movimientos involuntarios, agitación, rigidez muscular y contracciones musculares) provocados por otros medicamentos.
Niños y adolescentes
La experiencia de uso de Stalevo en pacientes de menos de 18 años es limitada. Por tanto, no se recomienda su administración en niños o adolescentes.
Toma de Stalevo con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
No tome Stalevo si está tomando determinados medicamentos para tratar la depresión (combinaciones de inhibidores selectivos de la MAO-A y MAO-B, o inhibidores no selectivos de la MAO).
Stalevo puede aumentar el efecto, y también los efectos adversos, de ciertos medicamentos. Entre ellos están:
- los medicamentos utilizados para tratar la depresión, como moclobemida, amitriptilina, desipramina, maprotilina, venlafaxina y paroxetina
- rimiterol e isoprenalina, empleados para tratar las enfermedades respiratorias
- adrenalina, utilizada para las reacciones alérgicas graves
- noradrenalina, dopamina y dobutamina, que se usan para tratar las enfermedades cardíacas y la tensión arterial baja
- alfa‑metildopa, empleada para tratar la tensión arterial alta
- apomorfina, utilizada para tratar la enfermedad de Parkinson.
Determinados medicamentos pueden reducir los efectos de Stalevo. Estos medicamentos son
- Antagonistas de la dopamina empleados para tratar trastornos mentales, las náuseas y los vómitos
- La fenitoína, empleada para prevenir las convulsiones
- La papaverina, empleada para relajar los músculos.
Stalevo puede dificultar la absorción del hierro a nivel digestivo. Por consiguiente, no debe tomar Stalevo a la misma vez que toma suplementos de hierro. Si toma alguno de ellos, debe esperar al menos de 2 ó 3 horas antes de tomar el otro.
Toma de Stalevo con alimentos y bebidas
- Stalevo puede tomarse con o sin alimentos.
- Puede que en algunos pacientes Stalevo no se absorba bien si se toma con alimentos ricos en proteínas (carnes, pescados, lácteos, cereales y frutos secos) o poco después de ingerirlos. Consulte a su médico si cree que esto ocurre en su caso.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de tomar este medicamento.
No debe dar el pecho a su hijo durante el tratamiento con Stalevo.
Conducción y uso de máquinas
Stalevo puede reducir su presión arterial, lo que puede producirle mareos o desvanecimientos. Por consiguiente, tenga especial cuidado al manejar herramientas o maquinaria.
Si se nota muy cansado u observa que en ocasiones se queda dormido de repente, espere hasta que se sienta totalmente despierto de nuevo antes de conducir o hacer cualquier otra cosa que le exija estar alerta. Si no, puede ponerse a sí mismo y a otros en peligro de sufrir un accidente grave o incluso mortal.
Stalevo contiene sacarosa
Stalevo contiene sacarosa (1,4 mg/comprimido). Si su el médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar Stalevo
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacáutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos y edad avanzada:
- Su médico le indicará el número exacto de comprimidos de Stalevo que debe tomar al día.
- Los comprimidos no deben partirse ni dividirse en trozos más pequeños.
- Tome solo un comprimido cada vez.
- Dependiendo de cómo responda al tratamiento, puede que su médico le indique que aumente o reduzca la dosis.
- Si está tomando comprimidos de Stalevo de 50 mg/12,5 mg/200 mg, 75 mg/18,75 mg/200 mg, 100 mg/25 mg/200 mg, 125 mg/31,25 mg/200 mg o 150 mg/37,5 mg/200 mg, no tome más de 10 comprimidos al día.
Hable con su médico o farmacéutico si cree que el efecto de Stalevo es demasiado intenso o demasiado débil, o si sufre posibles efectos adversos.
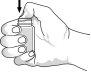
Para abrir el frasco la primera vez: abra el cierre presionando con el dedo pulgar en el sello hasta que se rompa. Ver Figura 1. | Figura 1
|
Si toma más Stalevo del que debe
Si accidentalmente ha tomado más comprimidos de Stalevo de los que debiera, hable inmediatamente con su médico o farmacéutico. En caso de sobredosis, es posible que se sienta aturdido o agitado, que el ritmo del corazón sea más lento o más rápido de lo normal, o que cambie el color de su piel, lengua, ojos u orina.
Si olvidó tomar Stalevo
No tome una dosis doble para compensar las dosis olvidadas.
Si falta más de 1 hora hasta la próxima dosis:
Tome un comprimido en cuanto se acuerde, y el siguiente a la hora normal.
Si falta menos de 1 hora hasta la próxima dosis:
Tome un comprimido en cuanto se acuerde, espere 1 hora y tome otro comprimido entonces. A continuación, siga como de costumbre.
Siempre debe transcurrir una hora entre comprimidos de Stalevo, para evitar posibles efectos adversos.
Si interrumpe el tratamiento con Stalevo
No deje de tomar Stalevo a menos que su médico así se lo indique. En ese caso, es posible que su médico tenga que ajustar la dosis de los demás medicamentos contra el Parkinson, especialmente de levodopa, para que el control de sus síntomas sea suficiente. Si deja de tomar Stalevo y otros medicamentos contra el Parkinson de forma brusca, puede sufrir efectos adversos no deseados.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Muchos se pueden aliviar ajustando la dosis.
Si experimenta alguno de estos síntomas durante el tratamiento con Stalevo, póngase en contacto con su médico de inmediato:
- Gran rigidez o contracciones violentas de los músculos, temblores, agitación, confusión, fiebre, pulso rápido o grandes fluctuaciones de la presión arterial. Pueden ser síntomas de síndrome neuroléptico maligno (SNM, una rara reacción grave a los medicamentos utilizados para tratar los trastornos del sistema nervioso central) o de rabdomiolisis (una alteración muscular rara y grave).
- Reacción alérgica caracterizada por signos como urticaria, picor, erupción e hinchazón de la cara, los labios, la lengua o la garganta. Esta reacción puede causar dificultades para respirar o para tragar.
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- movimientos incontrolados (discinesias)
- sensación de malestar (náuseas)
- coloración marrón rojiza de la orina inocua
- dolor muscular
- diarrea
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- desvanecimiento o desmayo por disminución de la presión arterial, hipertensión
- empeoramiento de los síntomas del parkinsonismo, mareos, somnolencia
- vómitos; dolor y malestar abdominal; ardor de estómago; sequedad de boca; estreñimiento
- incapacidad de dormir; alucinaciones; confusión; sueños anómalos (con pesadillas); cansancio
- cambios del estado mental, incluidos problemas de memoria, ansiedad y depresión (posiblemente con pensamientos de suicidio)
- dolencias cardíacas o arteriales (p. ej., dolor torácico), irregularidades de la frecuencia cardiaca
- caídas más frecuentes
- respiración fatigosa
- aumento de la sudoración, erupciones
- calambres musculares, hinchazón de las piernas
- visión borrosa
- anemia
- disminución del apetito, pérdida de peso
- dolor de cabeza, dolor en las articulaciones
- infecciones urinarias
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- infarto
- hemorragia intestinal
- alteraciones del número de células de la sangre que pueden provocar hemorragias; anomalías de las pruebas de la función hepática
- convulsiones
- sensación de agitación
- síntomas psicóticos
- colitis (inflamación del colon)
- cambios de color distintos de los de la orina (p.ej., piel, uñas, cabello, sudor)
- dificultad para tragar
- incapacidad para orinar
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
Deseo de grandes dosis de Stalevo superiores a las requeridas para controlar los síntomas motores, lo que se conoce como síndrome de disregulación de dopamina. Algunos pacientes experimentan movimientos involuntarios anormales graves (discinesias), cambios de humor u otros efectos adversos después de tomar grandes dosis de Stalevo.
Se han notificado también los efectos adversos siguientes:
- hepatitis (inflamación del hígado)
- picor
Usted puede experimentar los siguientes efectos adversos:
- Incapacidad de resistir el impulso de realizar una acción que podría resultar perjudicial, y que podría incluir:
- un fuerte impulso de jugar excesivamente a pesar de las consecuencias graves personales como familiares
- un incremento o alteración del interés sexual y un comportamiento preocupante para usted o para los demás, como por ejemplo, una tendencia sexual aumentada
- compras o gastos excesivos e incontrolados
- comer de forma excesiva (comer grandes cantidades de comida en periodos de tiempo cortos) o compulsiva (comer más comida de la habitual y más de lo que es necesario para satisfacer el apetito).
Informe a su médico si presenta alguno de estos comportamientos: ellos discutirán la manera de manejar o reducir estos síntomas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Anexo V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Stalevo
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases o de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Stalevo
- Los principios activos de Stalevo son levodopa, carbidopa y entacapona.
- Cada comprimido de Stalevo 75 mg/18,75 mg/200 mg contiene 75 mg de levodopa, 18,75 mg de carbidopa y 200 mg de entacapona.
- Los demás componentes del núcleo del comprimido son croscarmelosa sódica, estearato de magnesio, almidón de maíz, manitol (E 421) y povidona (E 1201)
- Los componentes de la cubierta pelicular son glicerol 85 por ciento (E 422), hipromelosa, estearato de magnesio, polisorbato 80, óxido de hierro rojo (E 172), sacarosa y dióxido de titanio (E 171).
Aspecto del producto y contenido del envase
Stalevo 75 mg/18,75 mg/200 mg: comprimidos de color rojo parduzco claro, ovales, con “LCE 75” grabado en una de las caras.
Hay cinco tamaños diferentes de envase de Stalevo 75 mg/18,75 mg/200 mg (de 10, 30, 100, 130 o 175 comprimidos). Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Orion Corporation
Orionintie 1
FI‑02200 Espoo
Finlandia
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
Belgique/België/Belgien Orion Pharma BVBA/SPRL Tél/Tel: +32 (0)15 64 10 20 | Lietuva UAB Orion Pharma Tel. +370 5 276 9499 |
???????? Orion Corporation ???.: +358 10 4261 | Luxembourg/Luxemburg Orion Pharma BVBA/SPRL Tél/Tel: +32 (0)15 64 10 20 |
Ceská republika Orion Pharma s.r.o. Tel: +420 234 703 305 | Magyarország Orion Pharma Kft. Tel.: +36 1 239 9095 |
Danmark Orion Pharma A/S Tlf: +45 8614 0000 | Malta Orion Corporation Tel: +358 10 4261 |
Deutschland Orion Pharma GmbH Tel: +49 40 899 6890 | Nederland Orion Pharma BVBA/SPRL Tél/Tel: +32 (0)15 64 10 20 |
Eesti Orion Pharma Eesti OÜ Tel: +372 66 44 550 | Norge Orion Pharma AS Tlf: +47 40 00 42 10 |
Ελλ?δα Orion Pharma Hellas M.E.Π.E Τηλ: + 30 210 980 3355 | Österreich Orion Pharma GmbH Tel: +49 40 899 6890 |
España Orion Pharma S.L. Tel: + 34 91 599 86 01 | Polska Orion Pharma Poland Sp z.o.o. Tel.: + 48 22 8333177 |
France Centre Spécialités Pharmaceutiques Tel: + 33 (0) 1 47 04 80 46 | Portugal Orionfin Unipessoal Lda Tel: + 351 21 154 68 20 |
Hrvatska Orion Pharma d.o.o. Tel: +386 (0) 1 600 8015 | România Orion Corporation Tel: +358 10 4261 |
Ireland Orion Pharma (Ireland) Ltd. c/o Allphar Services Ltd. Tel: +353 1 428 7777 | Slovenija Orion Pharma d.o.o. Tel: +386 (0) 1 600 8015 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Orion Pharma s.r.o Tel: +420 234 703 305 |
Italia Orion Pharma S.r.l. Tel: + 39 02 67876111 | Suomi/FinlandOrion Corporation Puh./Tel: +358 10 4261 |
Κ?προς Lifepharma (ZAM) Ltd Τηλ.: +357 22056300 | Sverige Orion Pharma AB Tel: +46 8 623 6440 |
Latvija Orion Corporation Orion Pharma parstavnieciba Tel: +371 20028332 | United Kingdom Orion Pharma (UK) Ltd. Tel: +44 1635 520 300 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu
- País de registro
- Precio medio en farmacia74.71 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a STALEVO 75 mg/18,75 mg/200 mg COMPRIMIDOS RECUBIERTOS CON PELICULAForma farmacéutica: GEL INTESTINAL, 20 mg/ml + 5 mg/ml + 20 mg/mlPrincipio activo: levodopa, decarboxylase inhibitor and COMT inhibitorFabricante: Lobsor Pharmaceuticals AbRequiere recetaForma farmacéutica: COMPRIMIDO, 100 mg/25 mg/200 mgPrincipio activo: levodopa, decarboxylase inhibitor and COMT inhibitorFabricante: Abdi Farma GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 125mg/31.25mg/200 mgPrincipio activo: levodopa, decarboxylase inhibitor and COMT inhibitorFabricante: Abdi Farma GmbhRequiere receta
Médicos online para STALEVO 75 mg/18,75 mg/200 mg COMPRIMIDOS RECUBIERTOS CON PELICULA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de STALEVO 75 mg/18,75 mg/200 mg COMPRIMIDOS RECUBIERTOS CON PELICULA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes