RIVASTIGMINA VIATRIS 9,5 MG/24H PARCHES TRANSDÉRMICOS EFG

Cómo usar RIVASTIGMINA VIATRIS 9,5 MG/24H PARCHES TRANSDÉRMICOS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
RivastigminaViatris9,5 mg/24 h parchestransdérmicos EFG
rivastigmina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Rivastigmina Viatris y para qué se utiliza
- Qué necesita saber antes de empezar a usar Rivastigmina Viatris
- Cómo usar Rivastigmina Viatris
- Posibles efectos adversos
- Conservación de Rivastigmina Viatris
- Contenido del envase e información adicional
1. Qué es Rivastigmina Viatris y para qué se utiliza
El principio activo de Rivastigmina Viatris es rivastigmina.
Rivastigmina pertenece al grupo de los inhibidores de la colinesterasa. En pacientes con demencia de Alzheimer, determinadas células nerviosas mueren en el cerebro, provocando bajos niveles de neurotransmisores de acetilcolina (una substancia que permite que las células nerviosas se comuniquen entre ellas). Rivastigmina actúa bloqueando las enzimas que rompen la acetilcolina: acetilcolinesterasa y butirilcolinesterasa.
Bloqueando estas enzimas, rivastigmina permite el aumento de acetilcolina en el cerebro, ayudando a reducir los síntomas de la enfermedad de Alzheimer.
Rivastigmina Viatris se utiliza para el tratamiento de pacientes adultos con demencia de Alzheimer de leve a moderadamente grave, un trastorno progresivo del cerebro que afecta gradualmente a la memoria, capacidad intelectual y el comportamiento.
2. Qué necesita saber antes de empezar a usar Rivastigmina Viatris
No use Rivastigmina Viatris
- Si es alérgico a rivastigmina (el principio activo de Rivastigmina Viatris) o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si alguna vez ha tenido una reacción alérgica a un medicamento similar (derivados del carbamato).
- Si tiene una reacción en la piel que se extiende más allá del tamaño del parche, si hubo una reacción local más intensa (tales como ampollas, inflamación de la piel en aumento, hinchazón) y si no mejora durante las 48 horas después de retirar el parche transdérmico.
Si se encuentra en algunas de estas situaciones, informe a su médico y no utilice Rivastigmina Viatris.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Rivastigmina Viatris:
- Si tiene, o ha tenido alguna vez el ritmo cardíaco (pulso) irregular o lento.
- Si tiene insuficiencia cardíaca.
- Si ha sufrido un ataque al corazón.
- Si tiene, o ha tenido alguna vez, los niveles bajos de potasio o magnesio en sangre.
- Si tiene, o ha tenido alguna vez, una úlcera de estómago activa.
- Si tiene, o ha tenido alguna vez, dificultades al orinar.
- Si tiene, o ha tenido alguna vez, convulsiones.
- Si tiene, o ha tenido alguna vez, asma o una enfermedad respiratoria grave.
- Si sufre temblores.
- Si tiene peso corporal bajo.
- Si tiene reacciones gastrointestinales tales como sensación de mareo (náuseas), malestar general (vómitos) y diarrea. Podría deshidratarse (pérdida de gran cantidad de fluido) si los vómitos o diarrea son prolongados.
- Si tiene insuficiencia hepática.
Si se encuentra en alguna de estas situaciones, puede que su médico considere necesario realizar un mayor seguimiento mientras esté en tratamiento.
Niños y adolescentes
No existe experiencia del uso de Rivastigmina Viatris en la población pediátrica en el tratamiento de la enfermedad de Alzheimer.
Uso de Rivastigmina Viatris con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta médica.
Rivastigmina Viatris podría incrementar los efectos de algunos medicamentos que disminuyen la presión arterial; por ejemplo, "betabloqueantes", como atenolol; "antagonistas de los canales de calcio", como amlodipino, nifedipino; "medicamentos antiarrítmicos", como sotalol, amiodarona, digitálicos y pilocarpina (usados para tratar el glaucoma). Esto podría ocasionarle un desmayo.
En caso de que se le administren algunos de los medicamentos siguientes, su médico puede someterle a revisiones cardiacas regulares para asegurarse de que no presenta problemas:
- Medicamentos usados para tratar problemas de salud mental conocidos como "antipsicóticos"; por ejemplo, clorpromazina, levomepromazina, sulpirida, tiaprida, veraliprida, pimozida, haloperidol, droperidol.
- Un medicamento conocido como cisaprida (usado para tratar la indigestión).
- Citalopram (utilizado para tratar la depresión).
- Difemanilo (usado para tratar la úlcera péptica).
- Halofrantina (usada para tratar la malaria).
- Mizolastina (usada para tratar las alergias).
- Metadona (un analgésico, también usado en la adicción a la heroína).
- Eritromicina IV, pentamidina, moxifloxacino (antibióticos).
Rivastigmina podría interferir con medicamentos anticolinérgicos algunos de los cuales son medicamentos utilizados para aliviar los calambres o espasmos estomacales (p.ejemplo. diciclomina), para el tratamiento de la enfermedad de Parkinson (p.ejemplo. amantadina), para tratar una vejiga hiperactiva (por ejemplo, oxibutinina, tolterodina) o para prevenir los mareos por movimiento (p.ejemplo. difenhidramina, escopolamina, o meclizina).
Rivastigmina Viatris no se debe administrar al mismo tiempo que metoclopramida (un medicamento utilizado para aliviar o prevenir las náuseas y los vómitos). La toma de los dos medicamentos juntos puede causar problemas como rigidez en las extremidades y temblor de manos.
En caso de que tenga que someterse a una intervención quirúrgica mientras está utilizando Rivastigmina Viatris, informe a su médico de que lo está utilizando, ya que puede necesitar detenerlo porque puede potenciar excesivamente los efectos de algunos relajantes musculares de la anestesia.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Si está embarazada es necesario evaluar los beneficios del uso de Rivastigmina Viatris frente a los posibles efectos adversos para el feto. No se debe utilizar Rivastigmina Viatris durante el embarazo a menos que sea claramente necesario.
No debe dar el pecho durante su tratamiento con Rivastigmina Viatris.
Conducción y uso de máquinas
Su enfermedad puede afectar la capacidad de conducir o utilizar maquinaria y no debe llevar a cabo estas actividades a menos que su médico le indique que es seguro realizarlas. Rivastigmina Viatris puede causar mareos y somnolencia, principalmente al inicio del tratamiento o al aumentar la dosis. Si experimenta estos efectos, no debe conducir o utilizar maquinaria.
3. Cómo usar Rivastigmina Viatris
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda consulte a su médico o farmacéutico.
IMPORTANTE
- Quitar el parche anterior antes de ponerse UN parche nuevo.
- Se debe poner solo un parche deRivastigmina Viatris al día.
- No corte el parche en trozos.
- Presionar firmemente el parche contra la piel con la palma de la mano durante un mínimo de 30 segundos.
- Se debe evitar tocar los ojos tras manipular el parche.
Cómo iniciar el tratamiento
Su médico le indicará la dosis de Rivastigmina Viatris parche transdérmico más adecuada en su caso.
- Normalmente se comienza el tratamiento con Rivastigmina Viatris 4,6 mg/24 h.
- La dosis diaria habitual recomendada es Rivastigmina Viatris 9,5 mg/24 h. Si su estado no mejora después de al menos 6 meses, su médico puede considerar aumentar la dosis a 13,3 mg/24 h (Este medicamento no tiene la concentración de 13,3 mg/24 horas. Para las situaciones en las que deba usar esta dosis, consulte con su farmacéutico).
- Lleve solo un parche transdérmico al mismo tiempo y sustituya el parche por otro nuevo a las 24 horas.
Durante el tratamiento, su médico podría ajustar la dosis dependiendo de sus necesidades individuales.
Si no ha utilizado los parches durante tres días, no se ponga otro antes de que lo haya consultado a su médico ya que puede ser más propenso a experimentar efectos adversos. El tratamiento con parche transdérmico se puede reiniciar con la misma dosis si el tratamiento no se interrumpe durante más de tres días. De lo contrario, su médico le hará reiniciar su tratamiento con Rivastigmina Viatris 4,6 mg/24 h.
Dónde colocar Rivastigmina Viatris parches transdérmicos
- Antes de ponerse un parche, asegúrese que la piel esté limpia, seca y sin pelo, sin polvos, aceite, hidratante o loción que impidan que el parche se pegue bien a la piel, sin cortes, enrojecimientos o irritaciones.
- Quitar cuidadosamente cualquier parche que lleve antes de poner uno nuevo.Llevar múltiples parches en su cuerpo podría exponerlo a una cantidad excesiva de este medicamento y esto podría ser potencialmente peligroso.
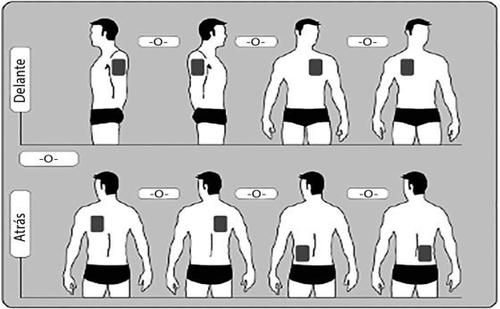
- Poner solo UNparche al día en UNA SOLAde las siguientes zonas como se muestra en los siguientes diagramas:
- Parte superior del brazo derecho oizquierdo.
- Parte superior izquierda del pecho oparte superior derecha del pecho (evitando los senos).
- Parte superior izquierda de la espalda oparte superior derecha de la espalda.
- Parte inferior izquierda de la espalda oparte inferior derecha de la espalda.
Cada 24 horas quitar el parche previo antes de poner UN parche nuevo en SOLO UNA de las siguientes zonas posibles.
|
Cada vez que se cambie el parche, se debe quitar el parche del día anterior antes de ponerse el nuevo parche en un lugar diferente de la piel (por ejemplo un día en el lado derecho del cuerpo y al día siguiente en el lado izquierdo; y un día en el pecho o en la parte superior de la espalda y al día siguiente en la parte inferior de la espalda). Esperar al menos 14 días para volver a poner un parche nuevo exactamente en la misma zona de piel.
Cómo aplicar Rivastigmina Viatris
Los parches de Rivastigmina Viatris son de plástico fino, de color canela, y se pegan a la piel. Cada parche se encuentra en un sobre que lo protege hasta que se lo vaya a poner. No abra el sobre ni saque el parche del sobre hasta el momento de ponérselo.
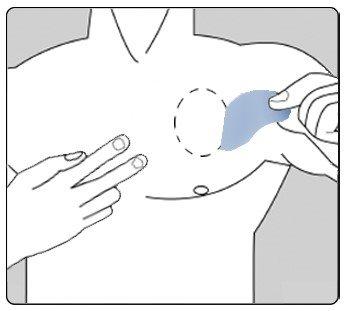
Se debe quitar cuidadosamente el parche existente antes de ponerse uno nuevo.
Los pacientes que inician el tratamiento por primera vez y para pacientes que reinician el tratamiento con rivastigmina después de la interrupción del tratamiento, deben empezar por la segunda figura.
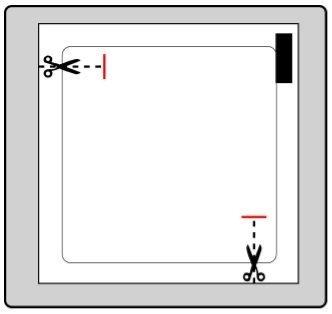
Cada parche se encuentra en un sobre protector individual.
Solo se debe abrir el sobre cuando vaya a ponerse el parche.
Corte el sobre por ambas marcas de tijeras, sin superar la línea indicada. Rasgue el sobre para abrirlo. No corte toda la longitud del sobre, para evitar que el parche se dañe.
Saque el parche del sobre.
Retire la hoja de cobertura, del color de la piel, de la parte superior del parche y deséchela.
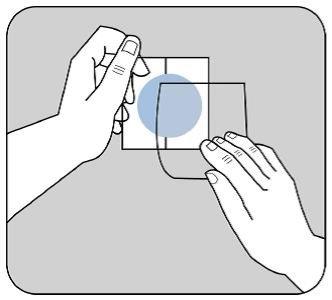
Una lámina protectora cubre el lado adhesivo del parche.
Quite la primera hoja de la lámina sin tocar con los dedos el lado adhesivo del parche.
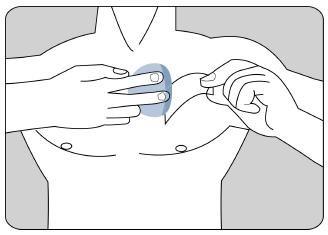
Coloque el lado adhesivo del parche sobre la parte superior o inferior de la espalda o en la parte superior del brazo o en el pecho y a continuación quite la segunda hoja de la lámina protectora.
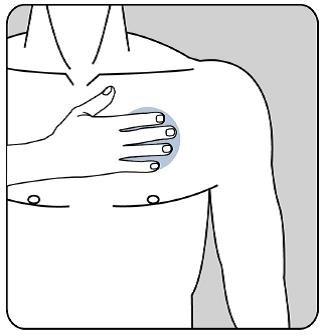
Después presione firmemente el parche contra la piel con la palma de la mano durante un mínimo de 30 segundos y asegúrese de que los bordes se han pegado bien.
Si esto le ayuda, puede escribir sobre el parche, por ejemplo, el día de la semana, con un bolígrafo de punta fina redondeada.
Debe llevar puesto el parche continuamente hasta el momento de cambiarlo por otro nuevo. Cuando se ponga un nuevo parche, puede probar con diferentes zonas (escogiendo entre las mencionadas previamente) para encontrar las que le resulten más cómodas y donde la ropa no roce con el parche.
Cómo quitar Rivastigmina Viatris
Tire suavemente de uno de los bordes del parche para despegarlo lentamente de la piel. Si quedan residuos adhesivos sobre la piel, empape el área con agua tibia y jabón suave o utilice aceite de bebé para ayudar a eliminarlos. No se debe utilizar alcohol u otros líquidos disolventes (quitaesmaltes de uñas u otros disolventes).
Lavar las manos
Lavar las manos con agua y jabón después de retirar o aplicar el parche. En caso de contacto con los ojos o si los ojos se enrojecen después de manipular el parche, se debe lavar inmediatamente con abundante agua y pedir consejo médico si los síntomas no se resuelven.
¿Se puede llevar Rivastigmina Viatris cuando se bañe, nade o se exponga al sol?
- El baño, la natación o la ducha no deberían afectar al parche. Se debe asegurar de que no se despegue parcialmente mientras realice estas actividades.
- No exponga al parche a una fuente de calor externa (por ejemplo luz solar excesiva, sauna, solárium) durante largos periodos de tiempo.
¿Qué debe hacer si se le cae un parche?
Si se le cayera un parche, se debe poner uno nuevo para el resto de ese día y cámbielo al día siguiente a la hora habitual.
¿Cuándo y durante cuánto tiempo debe ponerse Rivastigmina Viatris?
- Para beneficiarse de su tratamiento debe ponerse un nuevo parche cada día, preferiblemente a la misma hora.
- Lleve solo un parche de Rivastigmina Viatris al mismo tiempo y sustituya el parche por otro nuevo a las 24 horas.
Si usa más Rivastigmina Viatris del que debe
Si accidentalmente se ha puesto más de un parche, quite todos los parches de la piel e informe de ello a su médico o farmacéutico, o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 (indicando el medicamento y la cantidad administrada). Es posible que necesite atención médica.
Algunas personas que han usado por error demasiada rivastigmina han experimentado una disminución del tamaño de la pupila (miosis), enrojecimiento de la piel y sensación de calor (sofocos), dolor estomacal, sensación de malestar (náuseas), malestar (vómitos), diarrea, ritmo cardíaco lento, dificultad repentina al respirar (broncoespasmos), aumento de la producción de esputos, aumento de la sudoración, incontinencia o pérdida del control intestinal, llantos, hipotensión arterial, aumento de la salivación, mareos, temblores, cefaleas, sensación de sueño, confusión, presión arterial elevada, alucinaciones o falta de energía (malestar general). En casos graves, se ha documentado debilidad muscular, tics musculares incontrolables, ataques y paradas respiratorias o respiración más lenta.
Si olvidó usar Rivastigmina Viatris
Si se da cuenta que ha olvidado ponerse un parche, póngaselo inmediatamente. Al día siguiente póngase el siguiente parche a la hora habitual. No se ponga dos parches para compensar el que olvidó.
Si interrumpe el tratamiento con Rivastigmina Viatris
Informe a su médico o farmacéutico si deja de utilizar los parches.
Si no ha utilizado los parches durante tres días o más, no se ponga otro antes de que lo haya consultado a su médico ya que es más probable que experimente efectos adversos.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede tener efectos adversos con más frecuencia al empezar su tratamiento o cuando su dosis sea aumentada. Generalmente, los efectos adversos lentamente desaparecerán a medida que su organismo vaya acostumbrándose al medicamento.
Si advierte alguno de los siguientes efectos adversos que pueden ser graves, quítese el parche e informe inmediatamente a su médico:
- Crisis epilépticas (convulsiones).
- Cambios de ritmo del corazón, que tanto puede ser una frecuencia cardíaca muy rápida como una sensación de falta de latidos.
- Úlcera de estómago (puede que experimente dolor estomacal y si vomita puede aparecer sangre o restos similares a granos de café).
- Inflamación del páncreas – los signos incluyen dolor severo de la parte alta del estómago frecuentemente con sensación de mareo (náuseas) o mareo (vómitos).
- Sensación de sentirse muy confuso, que se puede asociar con ver, sentir u oír cosas que no existen (alucinaciones), sensación de alejarse de la realidad (ideas delirantes) actividad disminuida o aumentada (delirios).
- Alteraciones hepáticas (puede observar la coloración amarillenta de la piel o en el blanco de los ojos, oscurecimiento anómalo de la orina o náuseas sin explicación, vómitos, cansancio y pérdida del apetito).
Otros posibles efectos adversos podrían ser:
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Dolor de cabeza.
- Falta o pérdida del apetito, disminución del peso.
- Sensación de ansiedad, depresión, mareo.
- Desmayos.
- Sensación de malestar, malestar, diarrea, indigestión/ardor de estómago, dolor estomacal.
- Sensación de agitación, cansancio, debilidad general, febrícula.
- Erupciones cutáneas y reacciones cutáneas alérgicas donde se ha aplicado el parche, como reacciones similares a eczemas, enrojecimiento, prurito, hinchazón e irritación.
- Infección de orina (puede presentar dolor al orinar o necesidad de ir al baño con más frecuencia).
- Incontinencia urinaria (imposibilidad de detener adecuadamente la orina).
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Problemas cardíacos, tales como un ritmo de latidos lento.
- Deshidratación (pérdida de gran cantidad de fluido).
- Hiperactividad (alto nivel de actividad, inquietud).
- Agresividad.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- Caídas.
Muy raros(pueden afectar hasta 1 de cada 10.000 personas):
- Rigidez de piernas o brazos, inquietud, espasmos musculares, temblores (por ejemplo, en las manos).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Empeoramiento de los signos de la enfermedad de Parkinson; como temblores, rigidez, sensación de sueño y arrastrar los pies.
- Reacción cutánea alérgica, como ampollas o piel inflamada, prurito, urticaria o enrojecimiento.
- Latido rápido del corazón.
- Ver u oír cosas que en realidad no existen (alucinaciones).
- Tensión arterial alta.
- Cambios en los análisis que muestran el funcionamiento de su hígado.
- Sensación de inquietud.
- Pesadillas.
- Síndrome de Pisa (afección que conlleva una contracción muscular involuntaria y la inclinación anormal del cuerpo y la cabeza hacia un lado).
Otros efectos adversos experimentados con cápsulas o solución oral de rivastigmina y que pueden tener lugar con los parches:
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Sensación de malestar general.
- Sensación de confusión.
- Aumento de la sudoración.
Raros(pueden afectar hasta 1 de cada 1.000 personas):
- Dolor de pecho – causado probablemente por espasmos en el corazón.
- Úlcera en el intestino.
Muy raros(pueden afectar hasta 1 de cada 10.000 personas):
- Sangrado gastrointestinal – se manifiesta como sangre en las heces o al estar enfermo.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Algunas personas que han estado intensamente mareadas han tenido desgarro de parte del tubo digestivo que conecta su boca con su estómago (esófago).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de uso Humano, https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rivastigmina Viatris
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el sobre después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Conservar el parche transdérmico dentro del sobre hasta su uso.
No utilizar ningún parche si observa que está dañado o muestra signos de manipulación.
Tras quitarse un parche, dóblelo por la mitad con el lado adhesivo hacia dentro y presione. Tras introducirlo en el sobre original, al deshacerse del parche asegúrese de que quede fuera del alcance de los niños. Después de quitarse el parche no se toque los ojos, y lávese bien las manos con agua y jabón.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Rivastigmina Viatris
El principio activo es rivastigmina.
Cada parche libera 9,5 mg de rivastigmina en 24 horas, mide 9,2 cm2 y contiene 13,8 mg de rivastigmina.
Los demás componentes son:
Matriz:
- Poli [(2-etilhexil) acrilato, vinilacetato].
- Poliisobuteno de mediano y alto peso molecular.
- Sílice coloidal anhidra.
- Aceite de vaselina fluido.
Lámina de soporte:
- Lámina de polietileno/resina termoplástica/poliéster recubierto de aluminio.
Lámina de liberación:
- Película de poliéster recubierta de fluoropolímero.
- Tinta de impresión anaranjada.
Aspecto del producto y contenido del envase
Parche transdérmico fino. La capa externa es de color canela y lleva impreso en tinta de impresión anaranjada lo siguiente:
- "RIV-TDS 9,5 mg/24 h"
Cada sobre contiene un parche transdérmico. Los parches se encuentran disponibles en envases que contienen 7 o 30 sobres y en multienvases que contienen 60 o 90 sobres. Puede que solamente estén comercializados algunos tamaños de envases.
Titular dela autorización de comercializacióny responsable de la fabricación
Titular de la autorización de comercialización:
Viatris Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsable de la fabricación
McDermott Laboratories Ltd trading as Gerard Laboratories
35/36 Baldoyle Industrial Estate
Grange Road, Dublin 13
Irlanda
o
Mylan Hungary Kft
Mylan utca 1
Komárom, 2900
Hungría
o
Luye Pharma AG
Am Windfeld 27 and 35
83714 Miesbach
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Viatris Pharmaceuticals, S.L.U.
C/ General Aranaz, 86
28027 - Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania Rivastigmin Mylan 9.5 mg/24 Stunden transdermales Pflaster
Bélgica Rivastigmine Mylan 9.5 mg/24 h Pleisters voor transdermaal gebruik
España Rivastigmina Viatris 9.5 mg/24 h parches transdérmicos EFG
Francia Rivastigmine Mylan Pharma 9.5 mg/24 h Dispositif transdermique
Italia Rivastigmina Mylan Pharma 9.5 mg/24 h
Países Bajos Rivastigmin Pleister Mylan 9.5 mg/24 h Pleister voor transdermaal gebruik
Polonia Rivastigmine Mylan
Portugal Rivastigmina Mylan
Reino Unido Eluden 9.5 mg/24 h transdermal patch
Fecha de la última revisión de este prospecto:febrero 2025
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RIVASTIGMINA VIATRIS 9,5 MG/24H PARCHES TRANSDÉRMICOS EFGForma farmacéutica: PARCHE TRANSDERMICO, 13,3 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 4,6 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 9,5 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere receta
Médicos online para RIVASTIGMINA VIATRIS 9,5 MG/24H PARCHES TRANSDÉRMICOS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RIVASTIGMINA VIATRIS 9,5 MG/24H PARCHES TRANSDÉRMICOS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes