REMINYL 4 mg/ml SOLUCION ORAL

Cómo usar REMINYL 4 mg/ml SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Reminyl 4mg/ml solución oral
galantamina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4
Contenido del prospecto:
- Qué es Reminyl y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Reminyl
- Cómo tomar Reminyl
- Posibles efectos adversos
- Conservación de Reminyl
- Contenido del envase e información adicional
1. Qué es Reminyl y para qué se utiliza
Reminyl contiene el principio activo “galantamina”, un medicamento antidemencia. Se utiliza en adultos para tratar los síntomas de la enfermedad de Alzheimer de leve a moderadamente grave, un tipo de demencia que altera la función cerebral.
La enfermedad de Alzheimer provoca aumento de la pérdida de memoria, confusión y cambios de comportamiento, que hacen que sea cada vez más difícil realizar las actividades rutinarias de la vida cotidiana.
Se piensa que estos efectos son causados por una falta de “acetilcolina”, una sustancia responsable de la transmisión de mensajes entre las células del cerebro. Reminyl aumenta la cantidad de acetilcolina en el cerebro y de esta manera trata los signos de la enfermedad.
2. Qué necesita saber antes de empezar a tomar Reminyl
No tome Reminyl
- si es alérgico a galantamina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si padece una enfermedad de hígado o de riñón grave.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Reminyl. Este medicamento solo se debe utilizar en la enfermedad de Alzheimer y no se recomienda para otro tipo de pérdida de memoria o confusión.
Efectos adversos graves
Reminyl puede producir reacciones cutáneas graves, problemas en el corazón y convulsiones. Usted debe estar atento a estos efectos adversos mientras esté tomando Reminyl. Vea en la sección 4 “Esté atento a los efectos adversos graves”.
Antes de iniciar el tratamiento con Reminyl, su médico debe saber si tiene o ha tenido alguno de los siguientes trastornos:
- problemas de hígado o riñón
- un trastorno cardiaco (como malestar en el pecho que normalmente es causado por la actividad física, ataque cardiaco, insuficiencia cardiaca, latido del corazón lento o irregular, intervalo QTc prolongado)
- cambio en los niveles de electrolitos (sustancias químicas naturales en la sangre, como potasio)
- una úlcera péptica (estómago)
- obstrucción en el estómago o intestino
- un trastorno del sistema nervioso ([ tal como epilepsia o problemas en controlar los movimientos del cuerpo o extremidades (trastorno extrapiramidal) ])
- una enfermedad o infección respiratoria que afecta a la respiración (como asma, enfermedad pulmonar obstructiva o neumonía)
- problemas para la salida de orina.
Su médico decidirá si Reminyl es adecuado para usted o si es necesario cambiar la dosis.
Comente también con su médico si ha tenido recientemente una operaciónen el estómago, intestino o en la vejiga. Su médico decidirá si Reminyl es adecuado para usted.
Reminyl puede provocar una pérdida de peso.Su médico revisará su peso regularmente mientras esté tomando Reminyl.
Niños y adolescentes
No se recomienda el uso de Reminyl en niños ni en adolescentes.
Otros medicamentos y Reminyl
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Reminyl no debe tomarse junto con medicamentos que actúan de la misma manera. Éstos incluyen:
- donepezilo o rivastigmina (para la enfermedad de Alzheimer)
- ambenonio, neostigmina o piridostigmina (para la debilidad muscular grave)
- pilocarpina (cuando se toma por boca para la sequedad de ojos o boca).
Algunos medicamentos pueden producir efectos adversos con mayor probabilidad en personas que toman Reminyl. Éstos incluyen:
- medicamentos que afectan al intervalo QTc
- paroxetina o fluoxetina (antidepresivos)
- quinidina (para el latido cardiaco irregular)
- ketoconazol (un antifúngico)
- eritromicina (un antibiótico)
- ritonavir (para el virus de la inmunodeficiencia humana o “VIH”)
- analgésicos antiinflamatorios no esteroideos (como ibuprofeno), que pueden aumentar el riesgo de úlceras
- medicamentos para determinados trastornos del corazón o para la tensión alta (como digoxina, amiodarona, atropina, betabloqueantes o agentes bloqueantes de los canales de calcio). Si toma medicamentos debido a un latido irregular del corazón, su médico puede examinar su corazón mediante un “electrocardiograma” (ECG).
Si está tomando alguno de estos medicamentos, su médico puede darle una dosis más baja de Reminyl.
Reminyl puede afectar a algunos anestésicos. Si usted va a someterse a una operación bajo anestesia general, informe a su médico, con suficiente antelación, de que está tomando Reminyl.
Consulte a su médico o farmacéutico si tiene dudas.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe amamantar mientras esté tomando Reminyl.
Conducción y uso de máquinas
Reminyl puede hacer que se sienta mareado o soñoliento, especialmente durante las primeras semanas de tratamiento. Si Reminyl le afecta, no conduzca ni maneje herramientas o máquinas.
Reminyl solución oral contiene parahidroxibenzoatos de metilo y de propilo.
Estos algunas veces pueden producir reacciones alérgicas (posiblemente retardadas).
Reminyl solución oral contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 6 ml de Reminyl 4mg/ml solución oral (equivalente a la dosis máxima de Reminyl por día); esto es, esencialmente “exento de sodio”.
3. Cómo tomar Reminyl solución oral
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto tomar
Usted empezará el tratamiento con Reminyl a una dosis baja. La dosis inicial habitual es 4 mg (1 mL de solución), tomado dos veces al día (un total de 8 mg al día). Su médico le incrementará gradualmente su dosis, cada 4 semanas o más, hasta que alcance la dosis más adecuada para usted. La dosis máxima es 12 mg (3 ml de solución), tomada dos veces al día (un total de 24 mg al día).
Su médico le explicará con qué dosis debe empezar y cuándo debe aumentarla. Si no está seguro de qué hacer o encuentra que el efecto de Reminyl es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Su médico necesita verle de forma regular para comprobar que este medicamento está funcionando y comentar con usted cómo se siente.
Si tiene problemas de hígado o riñón,su médico puede darle una dosis reducida de Reminyl o puede decidir si este medicamento no es adecuado para usted.
Cómo tomarlo
Tome su dosis de Reminyl dos veces al día, por la mañana y por la noche, con agua u otros líquidos. Trate de tomar Reminyl con comida.
Beba líquido en abundancia mientras esté tomando Reminyl, para mantenerse hidratado.
La solución viene con una jeringuilla, con la que se puede extraer del frasco la cantidad exacta necesaria.
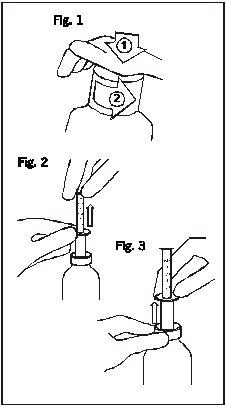
Instrucciones para abrir el frasco y utilizar la jeringuilla
Fig. 1: El frasco lleva un tapón de seguridad para niños y se debe abrir de la siguiente forma:
Fig. 2: Introduzca la jeringuilla en el frasco. Mientras sujeta el anillo inferior, tire del anillo superior hasta la marca correspondiente al número de mililitros que desea administrar. Fig. 3: Sujete el anillo inferior y retire la jeringuilla completa del frasco. Vacíe la jeringuilla en cualquier bebida no alcohólica deslizando el anillo superior hacia abajo y bébala inmediatamente. Cierre el frasco. Enjuague la jeringuilla con agua. |
|
Si toma más Reminyl del que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o
llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 indicando el medicamento y la
cantidad ingerida. Lleve consigo el envase con la solución que le haya quedado. Los signos de una sobredosis pueden incluir:
- náuseas intensas y vómitos
- debilidad muscular, latido lento del corazón, convulsiones y pérdida de consciencia.
Si olvidó tomar Reminyl
Si olvidó tomar una dosis, deje pasar esa dosis y continúe el tratamiento de la forma habitual con la toma de la siguiente dosis programada. No tome una dosis doble para compensar las dosis olvidadas.
Si olvida tomar más de una dosis, consulte a su médico.
Si interrumpe el tratamiento con Reminyl
Consulte con su médico antes de interrumpir el tratamiento con Reminyl. Es importante continuar tomando este medicamento para tratar su enfermedad.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Esté atento a los efectos adversos graves
Deje de tomar Reminyl y consulte a un médico o acuda al servicio de urgencias más cercano inmediatamentesi nota cualquiera de los siguientes efectos adversos
Reacciones de la piel,incluyendo:
- Erupción grave con ampollas y descamación de la piel, especialmente alrededor de la boca, nariz, ojos y genitales (síndrome de Stevens-Johnson).
- Erupción roja cubierta de pequeñas protuberancias llenas de pus que pueden propagarse por el cuerpo, a veces con fiebre (pustulosis exantemática generalizada aguda).
- Erupción que puede producir ampollas, con manchas que parecen pequeñas dianas.
Estas reacciones cutáneas son raras en las personas que toman Reminyl (pueden afectar hasta 1 de cada 1.000 personas).
Problemas del corazónincluyendo cambios del latido del corazón (como latido lento o latidos adicionales) o palpitaciones (sentir el latido del corazón rápido o irregular). Los problemas de corazón se pueden observar como un trazado anormal en un “electrocardiograma” (ECG), y puede ser común en las personas que toman Reminyl (pueden afectar hasta a 1 de cada 10 personas).
Convulsiones. Esto es poco frecuente en personas que toman Reminyl (pueden afectar hasta a 1 de cada 100 personas).
Debe dejar de tomar Reminyl y buscar ayuda inmediatamentesi nota alguno de los efectos adversos mencionados.
Otros efectos adversos
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Náuseas y vómitos. Estos efectos adversos son más probables que ocurran en las primeras semanas del tratamiento o cuando se eleva la dosis. Suelen desaparecer progresivamente a medida que el organismo se adapta al medicamento y por lo general solo duran unos pocos días. Si experimenta estos efectos, su médico le puede recomendar tomar más líquidos y puede prescribirle un medicamento para que no se sienta mal.
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- Disminución de apetito, pérdida de peso
- Ver, sentir u oír cosas que no están presentes (alucinaciones)
- Depresión
- Sensación de mareo o desmayo
- Temblores o espasmos musculares
- Dolor de cabeza
- Sentirse muy cansado, débil o malestar general
- Sensación de mucho sueño y tener poca energía
- Aumento de la presión arterial
- Dolor abdominal o malestar
- Diarrea
- Indigestión
- Caídas
- Heridas
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
- Reacción alérgica
- Insuficiente agua en el cuerpo (deshidratación)
- Hormigueo o entumecimiento de la piel
- Cambio del sentido del gusto
- Somnolencia diurna
- Problemas en controlar los movimientos del cuerpo o extremidades (trastorno extrapiramidal)
- Visión borrosa
- Pitido en los oídos que no desaparece (tinnitus)
- Presión arterial baja
- Rubor
- Sensación de necesidad de vomitar (arcadas)
- Sudoración excesiva
- Debilidad muscular
- Aumento del nivel de enzimas hepáticos en sangre.
Efectos adversos raros(pueden afectar hasta a 1 de cada 1.000 personas):
- Hígado inflamado (hepatitis).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es/. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Reminyl
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
No congelar.
La solución oral de Reminyl no se debe de utilizar una vez transcurridos 3 meses desde que se abrió el frasco por primera vez.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Reminyl
- El principio activo es galantamina. 1 mL de Reminyl solución oral contiene 4 mg de galantamina (como hidrobromuro).
- Los demás componentes son:
Parahidroxibenzoato de metilo (E218), parahidroxibenzoato de propilo (E216), agua purificada, sacarina sódica e hidróxido sódico.
Aspecto del producto y contenido del envase
Reminyl es una solución oral transparente e incolora que viene en un envase de 100 ml.
La solución viene con una jeringuilla con la que se puede extraer del frasco la cantidad exacta necesaria.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Essential Pharma Limited,
Vision Exchange Building,
Triq it-Territorjals, Zone 1,
Central Business District,
Birkirkara, CBD 1070,
Malta
Responsable de la fabricación
Janssen-Pharmaceutica, N.V.
Turnhoutseweg, 30
B-2340 Beerse
Bélgica
Este medicamento está autorizado en los Estados Miembros de la Unión Europea con los siguientes nombres:
Austria Reminyl 4 mg/ml- orale Lösung
Bélgica Reminyl 4 mg/ml, orale oplossing
Dinamarca Reminyl 4 mg/ml oral opløsning
Finlandia Reminyl 4 mg/ml oraaliliuos
Francia Reminyl 4 mg/ml, solution buvable
Grecia Reminyl 4 mg/ml π?σιμο δι?λυμα
Irlanda Reminyl 4 mg/ml oral solution
Italia Reminyl 4 mg/ml soluzione orale
- Luxemburgo Reminyl 4 mg/ml, soluté buvable
Noruega Reminyl 4 mg/ml mikstur, oppløsning
Portugal Reminyl 4 mg/ml solução oral
España Reminyl 4 mg/ml solución oral
Suecia Reminyl 4 mg/ml oral lösning
Reino Unido (Irlanda del Norte) Reminyl 4 mg/ml oral solution
Este prospecto ha sido aprobado en:octubre 2021.
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REMINYL 4 mg/ml SOLUCION ORALForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 16 mgPrincipio activo: galantamineFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 24 mgPrincipio activo: galantamineFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: CAPSULA LIBERACION MODIFICADA, 8 mgPrincipio activo: galantamineFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para REMINYL 4 mg/ml SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REMINYL 4 mg/ml SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes