
POMALIDOMIDA TEVA 4 MG CAPSULAS DURAS EFG

Cómo usar POMALIDOMIDA TEVA 4 MG CAPSULAS DURAS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Pomalidomida Teva 1 mg cápsulas duras
Pomalidomida Teva 2 mg cápsulas duras
Pomalidomida Teva 3 mg cápsulas duras
Pomalidomida Teva 4 mg cápsulas duras
pomalidomida
Se espera que Pomalidomida Teva cause graves defectos congénitos y que pueda ocasionar la muerte del feto.
|
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Pomalidomida Teva y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Pomalidomida Teva
- Cómo tomar Pomalidomida Teva
- Posibles efectos adversos
- Conservación de Pomalidomida Teva
- Contenido del envase e información adicional
1. Qué es Pomalidomida Teva y para qué se utiliza
Qué es Pomalidomida Teva
Pomalidomida Teva contiene el principio activo “pomalidomida”. Este medicamento está relacionado con la talidomida y pertenece a un grupo de medicamentos que afectan al sistema inmunitario (las defensas naturales del organismo).
Para qué se utiliza Pomalidomida Teva
Pomalidomida Teva se utiliza para tratar a adultos con un tipo de cáncer llamado “mieloma múltiple”.
Pomalidomida Teva se utiliza con:
- Otros dos medicamentosllamados “bortezomib” (un tipo de medicamento de quimioterapia) y “dexametasona” (un medicamento antiinflamatorio) en personas que han recibido al menos otro tratamiento, incluyendo lenalidomida.
U
- Otro medicamentollamado “dexametasona” en personas que han sufrido un empeoramiento de su mieloma, a pesar de haber recibido al menos otros dos tipos de tratamientos, incluyendo los medicamentos lenalidomida y bortezomib.
Qué es el mieloma múltiple
El mieloma múltiple es un tipo de cáncer que afecta a un tipo concreto de glóbulos blancos (denominados “células plasmáticas”). Estas células crecen sin control y se acumulan en la médula ósea, dañando los huesos y los riñones.
El mieloma múltiple generalmente no tiene cura. Sin embargo, el tratamiento puede reducir los signos y los síntomas de la enfermedad o hacerlos desaparecer durante un periodo de tiempo. Cuando esto ocurre, se le denomina “respuesta”.
Cómo actúa Pomalidomida Teva
Pomalidomida Teva actúa de diversas formas:
- detiene el desarrollo de las células del mieloma;
- estimula el sistema inmunitario para que ataque a las células cancerosas;
- detiene la formación de vasos sanguíneos que alimentan las células cancerosas.
Beneficio de utilizar Pomalidomida Teva con bortezomib y dexametasona
Si se utiliza Pomalidomida Teva con bortezomib y dexametasona en personas que han recibido al menos otro tratamiento, se puede detener la progresión del mieloma múltiple:
- Por lo general, la combinación de Pomalidomida Teva con bortezomib y dexametasona evitó la reaparición del mieloma múltiple durante un periodo de hasta 11 meses en comparación con los 7 meses observados en los pacientes que tomaban únicamente bortezomib y dexametasona.
Beneficio de utilizar Pomalidomida Teva con dexametasona
Si se utiliza Pomalidomida Teva con dexametasona en personas que han recibido al menos otros dos tratamientos, puede detener la progresión del mieloma múltiple:
- Por lo general, la combinación de Pomalidomida Teva y dexametasona evitó la reaparición del mieloma múltiple durante un periodo de hasta 4 meses en comparación con los 2 meses observados en los pacientes que tomaban dexametasona únicamente.
2. Qué necesita saber antes de empezar a tomar Pomalidomida Teva
No tome Pomalidomida Teva:
- si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, ya que se espera que Pomalidomida Teva sea perjudicial para el feto. (Los hombres y mujeres que estén tomando este medicamento deben leer la sección “Embarazo, anticoncepción y lactancia – información para mujeres y hombres” que aparece más abajo);
- si puede quedarse embarazada, a menos que esté tomando todas las medidas necesarias para evitar un embarazo (ver “Embarazo, anticoncepción y lactancia – información para mujeres y hombres”). Si puede quedarse embarazada, su médico anotará con cada receta que se han tomado todas las medidas necesarias y le proporcionará esta confirmación;
- si es alérgico a pomalidomida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si cree que podría ser alérgico, consulte a su médico.
Si no está seguro de si alguna de estas situaciones descritas es aplicable a usted, consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Pomalidomida Teva.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Pomalidomida Teva si:
- alguna vez ha tenido coágulos de sangre en el pasado. Durante el tratamiento con Pomalidomida Teva tiene un mayor riesgo de desarrollar coágulos de sangre en sus venas o arterias. Su médico le puede recomendar someterse a tratamientos adicionales (p. ej., warfarina) o reducir su dosis de Pomalidomida Teva para tener menos probabilidades de desarrollar coágulos sanguíneos;
- alguna vez ha sufrido una reacción alérgica, tales como erupción cutánea, picor, hinchazón, mareos o problemas respiratorios mientras tomaba medicamentos relacionados denominados “talidomida” o “lenalidomida”;
- ha sufrido un ataque al corazón, tiene insuficiencia cardiaca, tiene dificultad para respirar o, si es fumador, tiene la presión arterial alta o los niveles de colesterol altos;
- tiene una cantidad total de tumor alta en el cuerpo, incluida la médula ósea. Esto podría dar lugar a una enfermedad en la que los tumores se descomponen y producen niveles inusuales de sustancias químicas en la sangre que, a su vez, pueden originar insuficiencia renal. También puede experimentar latidos del corazón irregulares. Esta enfermedad se llama síndrome de lisis tumoral;
- sufre o ha sufrido neuropatía (daño neurológico que causa hormigueo o dolor en sus pies o sus manos);
- tiene o ha tenido infección por el virus de la hepatitis B. El tratamiento con Pomalidomida Teva puede volver a activar el virus de la hepatitis B en los pacientes portadores del virus, lo que da lugar a que la infección aparezca de nuevo (recurrencia). Su médico debe comprobar si alguna vez ha tenido una infección por el virus de la hepatitis B.
- experimenta o ha experimentado en el pasado una combinación de cualquiera de los síntomas siguientes: erupción en cara o generalizada, enrojecimiento de la piel, fiebre alta, síntomas de tipo gripal, nódulos linfáticos agrandados (síntomas de una reacción cutánea grave llamada reacción a fármacos con eosinofilia y síntomas sistémicos o síndrome de DRESS [por sus siglas en inglés] o síndrome de hipersensibilidad a fármacos, necrólisis epidérmica tóxica [NET] o síndrome de Stevens-Johnson [SSJ]. Ver también sección 4 “Posibles efectos adversos”).
Es importante señalar que los pacientes con mieloma múltiple tratados con pomalidomida pueden desarrollar otros tipos de cáncer, por lo que su médico debe evaluar cuidadosamente los beneficios y los riesgos al recetarle este medicamento.
En cualquier momento durante o después del tratamiento, informe a su médico o enfermero inmediatamente si: presenta visión borrosa, pérdida de la visión o visión doble, dificultad para hablar, debilidad en un brazo o una pierna, un cambio en la forma de caminar o problemas de equilibrio, entumecimiento persistente, disminución de la sensibilidad o pérdida de sensibilidad, pérdida de memoria o confusión. Todos ellos pueden ser síntomas de una enfermedad cerebral grave y potencialmente mortal conocida como leucoencefalopatía multifocal progresiva (LMP). Si tenía alguno de estos síntomas antes de empezar el tratamiento con Pomalidomida Teva, informe a su médico si observa algún cambio en estos síntomas.
Al final del tratamiento, debe devolver al farmacéutico todas las cápsulas sin usar.
Embarazo, anticoncepción y lactancia: información para hombres y mujeres
Debe seguir las siguientes indicaciones recogidas en el Programa de Prevención de Embarazo de Pomalidomida Teva. Los hombres y mujeres que estén tomando Pomalidomida Teva no deben engendrar hijos o quedarse embarazadas. El motivo es que se espera que pomalidomida sea perjudicial para el feto. Usted y su pareja deben usar métodos anticonceptivos eficaces mientras estén tomando este medicamento.
Mujeres
No tome Pomalidomida Teva si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada. El motivo es que se espera que este medicamento sea perjudicial para el feto. Antes de comenzar el tratamiento, debe informar a su médico si existe la posibilidad de que pueda quedarse embarazada, aunque crea que esto sea poco probable.
Si puede quedarse embarazada:
- debe usar métodos anticonceptivos eficaces desde, al menos, 4 semanas antes de iniciar el tratamiento, durante todo el tiempo que esté tomando el tratamiento y hasta, al menos,
- 4 semanas después de finalizarlo. Su médico le aconsejará sobre los métodos anticonceptivos más adecuados;
- cada vez que su médico le prescribe una receta, este se asegurará de que ha entendido las medidas necesarias que deben tomarse para prevenir el embarazo;
- su médico programará pruebas de embarazo antes del tratamiento, al menos, cada 4 semanas durante el tratamiento y, al menos, 4 semanas después de finalizar el tratamiento.
Si, a pesar de las medidas de prevención, se queda embarazada:
- debe suspender el tratamiento inmediatamente e informar a su médico de inmediato.
Lactancia
Se desconoce si Pomalidomida Teva pasa a la leche materna en humanos. Informe a su médico si está dando o si tiene intención de dar el pecho. Su médico le aconsejará si puede continuar o debe abandonar la lactancia.
Hombres
Pomalidomida Teva pasa al semen humano.
- Si su pareja está embarazada o puede quedarse embarazada, debe usar preservativos durante todo el tiempo que esté tomando el tratamiento y hasta 7 días después de finalizarlo.
- Si su pareja se queda embarazada mientras usted está tomando Pomalidomida Teva, informe a su médico inmediatamente. Su pareja también debe informar a su médico inmediatamente.
No debe donar semen o esperma durante el tratamiento y hasta 7 días después de finalizarlo.
Donación de sangre y análisis de sangre
No debe donar sangre durante el tratamiento y hasta 7 días después de haber finalizado el mismo. Antes de iniciar el tratamiento con Pomalidomida Teva y durante el mismo, le harán análisis de sangre periódicos. Esto se debe a que su medicamento puede provocar una disminución en el número de células sanguíneas que ayudan a luchar contra las infecciones (glóbulos blancos) y en el número de células que ayudan a parar el sangrado (plaquetas).
Su médico le pedirá que se haga un análisis de sangre:
- antes del tratamiento;
- cada semana durante las 8 primeras semanas de tratamiento;
- por lo menos una vez al mes mientras siga tomando Pomalidomida Teva.
Su médico puede ajustar la dosis de Pomalidomida Teva o interrumpir su tratamiento, dependiendo de los resultados de estas pruebas. Su médico también puede ajustar la dosis o interrumpir este medicamento debido a su estado de salud general.
Niños y adolescentes
No está recomendado el uso de Pomalidomida Teva en niños y adolescentes menores de 18 años de edad.
Otros medicamentos y Pomalidomida Teva
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto se debe a que Pomalidomida Teva puede afectar a la forma en que funcionan otros medicamentos. Además, algunos medicamentos pueden afectar a la forma en que funciona Pomalidomida Teva.
En particular, informe a su médico, farmacéutico o enfermero antes de tomar Pomalidomida Teva si está tomando alguno de los siguientes medicamentos:
- algunos antifúngicos como ketoconazol;
- algunos antibióticos (p. ej., ciprofloxacino, enoxacino);
- ciertos antidepresivos como fluvoxamina.
Conducción y uso de máquinas
Algunas personas experimentan cansancio, desmayos, confusión o disminución del estado de vigilia mientras toman Pomalidomida Teva. Si esto le ocurre a usted, no conduzca ni utilice herramientas o maquinaria.
Pomalidomida Teva contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por cápsula; esto es, esencialmente “exento de sodio”.
Pomalidomida Teva contiene lactosa
Si su médico la ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Pomalidomida Teva contiene azul brillante FCF (E133)
Este medicamento contiene el colorante azul brillante FCF (E133), que puede provocar reacciones alérgicas.
3. Cómo tomar Pomalidomida Teva
Pomalidomida Teva se lo debe administrar un médico con experiencia en el tratamiento del mieloma múltiple.
Siga exactamente las instrucciones de administración de los medicamentos indicadas por su médico. En caso de duda, consulte a su médico, farmacéutico o enfermero.
Cuándo tomar Pomalidomida Teva con otros medicamentos
Pomalidomida Teva en combinación con bortezomib y dexametasona
- Consulte el prospecto que se adjunta con bortezomib y dexametasona para obtener información adicional sobre su uso y sus efectos.
- Pomalidomida Teva, bortezomib y dexametasona se toman en ciclos de tratamiento. Cada ciclo dura 21 días (3 semanas).
- Observe el siguiente gráfico para consultar qué debe tomar cada día del ciclo de 3 semanas:
- Cada día observe el gráfico e identifique el día correcto para ver qué medicamentos debe tomar.
- Algunos días deberá tomar los 3 medicamentos, otros días solamente 1 o 2 medicamentos y otros días ninguno de ellos.
POM:Pomalidomida Teva; BOR:bortezomib; DEX:dexametasona
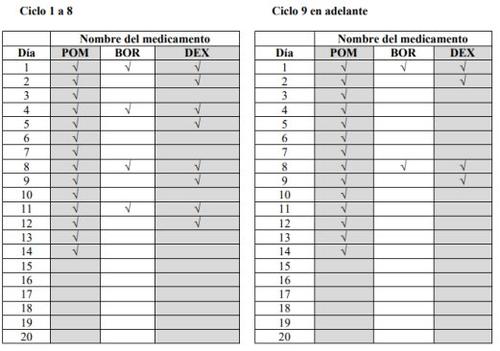
- Tras completar cada ciclo de 3 semanas, comience uno nuevo.
Pomalidomida Teva solo con dexametasona
- Consulte el prospecto que se adjunta con dexametasona para obtener información adicional sobre su uso y sus efectos.
- Pomalidomida Teva y dexametasona se toman en ciclos de tratamiento. Cada ciclo dura 28 días (4 semanas).
- Observe el siguiente gráfico para consultar qué debe tomar cada día del ciclo de 4 semanas:
- Cada día observe el gráfico e identifique el día correcto para ver qué medicamentos debe tomar.
- Algunos días deberá tomar ambos medicamentos, otros días solamente 1 medicamento y otros días ninguno de ellos.
POM:Pomalidomida Teva; DEX:dexametasona
Nombre del medicamento | ||
Día | POM | DEX |
1 | √ | √ |
2 | √ | |
3 | √ | |
4 | √ | |
5 | √ | |
6 | √ | |
7 | √ | |
8 | √ | √ |
9 | √ | |
10 | √ | |
11 | √ | |
12 | √ | |
13 | √ | |
14 | √ | |
15 | √ | √ |
16 | √ | |
17 | √ | |
18 | √ | |
19 | √ | |
20 | √ | |
21 | √ | |
22 | √ | |
23 | ||
24 | ||
25 | ||
26 | ||
27 | ||
28 |
- Tras completar cada ciclo de 4 semanas, comience uno nuevo.
Cuánta Pomalidomida Teva tomar con otros medicamentos
Pomalidomida Teva con bortezomib y dexametasona
- La dosis inicial recomendada de Pomalidomida Teva es de 4 mg al día.
- La dosis inicial recomendada de bortezomib será calculada por su médico según su altura y peso (1,3 mg/m2 de superficie corporal).
- La dosis inicial recomendada de dexametasona es de 20 mg al día. Sin embargo, si es usted mayor de 75 años de edad, la dosis inicial recomendada es de 10 mg al día.
Pomalidomida Teva solo con dexametasona
- La dosis recomendada de Pomalidomida Teva es de 4 mg una vez al día.
- La dosis inicial recomendada de dexametasona es de 40 mg al día. Sin embargo, si es usted mayor de 75 años de edad, la dosis inicial recomendada es de 20 mg al día.
Su médico puede tener que reducir la dosis de Pomalidomida Teva, bortezomib o dexametasona, o interrumpir uno o más de estos medicamentos en función de los resultados de su analítica de sangre y de su estado general, de si está tomando otros medicamentos (p. ej., ciprofloxacino, enoxacino y fluvoxamina) y si experimenta efectos adversos (especialmente erupción cutánea o hinchazón) como consecuencia del tratamiento.
Si usted sufre problemas hepáticos o renales, su médico realizará un cuidadoso seguimiento de su enfermedad mientras reciba este medicamento.
Cómo tomar Pomalidomida Teva
- No rompa, abra ni mastique las cápsulas. Si los polvos de una cápsula rota entran en contacto con la piel, lave la piel inmediatamente y abundantemente con agua y jabón.
- Los profesionales sanitarios, cuidadores y familiares se deben poner guantes desechables cuando manipulen el blíster o la cápsula. Posteriormente, se deben quitar los guantes con cuidado para evitar la exposición cutánea, introducirlos en una bolsa de plástico de polietileno sellable y eliminarlos de acuerdo con los requisitos locales. A continuación, se deben lavar bien las manos con agua y jabón. Las mujeres embarazadas o que sospechen que puedan estarlo no deben manipular el blíster ni la cápsula.
- Trague las cápsulas enteras, preferiblemente con agua.
- Puede tomar las cápsulas con o sin alimentos.
- Debe tomar las cápsulas aproximadamente a la misma hora cada día.
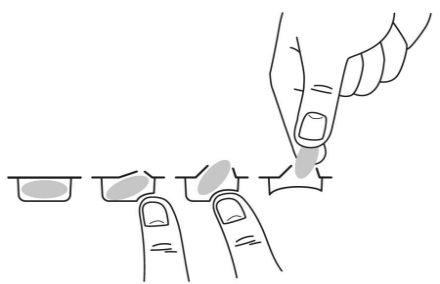
Para sacar la cápsula del blíster, presione solo un extremo de la cápsula para que salga a través de la lámina. No presione en el centro de la cápsula ya que podría romperla.
Su médico le aconsejará sobre cómo y cuándo tomar Pomalidomida Teva si tiene problemas renales y está recibiendo tratamiento con diálisis.
Duración del tratamiento con Pomalidomida Teva
Debe continuar los ciclos de tratamiento hasta que su médico le comunique que suspenda el tratamiento.
Si toma más Pomalidomida Teva del que debe
Si toma más Pomalidomida Teva del que debe, informe a su médico o acuda al hospital inmediatamente. Traiga el envase del medicamento con usted.
Si olvidó tomar Pomalidomida Teva
Si olvidó tomar Pomalidomida Teva el día que debía, tome la próxima cápsula al día siguiente a la hora habitual. No tome más cápsulas para compensar la dosis de Pomalidomida Teva que olvidó el día anterior.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Si experimenta alguno de los siguientes efectos adversos graves, interrumpa el tratamiento con Pomalidomida Teva y acuda a un médico inmediatamente, porque podría requerir tratamiento médico de urgencia:
- Fiebre, escalofríos, dolor de garganta, tos, úlceras bucales o cualquier otro signo de infección (debido a la disminución de glóbulos blancos que se ocupan de luchar frente a la infección).
- Hemorragia o moratones sin causa aparente, incluyendo hemorragias nasales y hemorragia intestinal o estomacal (debido a los efectos sobre las células sanguíneas llamadas “plaquetas”).
- Respiración rápida, pulso rápido, fiebre y escalofríos, capacidad para orinar escasa o inexistente, náuseas y vómitos, confusión, inconsciencia (debido a una infección de la sangre llamada sepsis o choque séptico).
- Diarrea grave, persistente o sanguinolenta (posiblemente acompañada de dolor de estómago o fiebre) causada por la bacteria Clostridium difficile.
- Dolor en el pecho o en las piernas e hinchazón, especialmente en la parte inferior de la pierna o las pantorrillas (producido por coágulos de sangre).
- Dificultad respiratoria (debido a una infección grave en el pecho, inflamación del pulmón, insuficiencia cardiaca o coágulos de sangre).
- Hinchazón de la cara, labios, lengua y garganta, que puede causar dificultad respiratoria (debido a unos tipos graves de reacciones alérgicas llamadas angioedema y reacción anafiláctica).
- Ciertos tipos de cáncer de piel (carcinoma de células escamosas y carcinoma de células basales), que pueden producir cambios en el aspecto de la piel o bultos en la piel. Si observa cambios en el aspecto de la piel mientras toma Pomalidomida Teva, informe a su médico lo antes posible.
- Recurrencia de la infección por el virus de la hepatitis B, que puede causar amarilleamiento de la piel y de los ojos, orina de color marrón oscuro, dolor abdominal en el lado derecho, fiebre, náuseas o malestar. Informe a su médico inmediatamente si observa alguno de estos síntomas.
- Erupción generalizada, temperatura corporal alta, nódulos linfáticos agrandados y efectos sobre otros órganos del cuerpo (reacción a fármacos con eosinofilia y síntomas sistémicos, que también se conoce como síndrome de DRESS o síndrome de hipersensibilidad a fármacos, necrólisis epidérmica tóxica o síndrome de Stevens-Johnson). Deje de tomar pomalidomida si presenta estos síntomas y póngase en contacto con su médico o acuda al médico inmediatamente. Ver también sección 2.
Si experimenta alguno de los siguientes efectos adversos graves, interrumpa el tratamiento con Pomalidomida Teva y acuda a un médico inmediatamente, porque podría requerir tratamiento médico de urgencia.
Otros efectos adversos
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Dificultad respiratoria (disnea).
- Infección de los pulmones (neumonía y bronquitis).
- Infecciones en la nariz, senos paranasales y garganta causadas por bacterias o virus.
- Síntomas de tipo gripal (gripe).
- Recuento bajo de glóbulos rojos, lo que puede producir anemia que da lugar a cansancio y debilidad.
- Niveles bajos de potasio en sangre (hipopotasemia), que puede causar debilidad, calambres y dolores musculares, palpitaciones, hormigueo o entumecimiento, disnea y cambios de humor.
- Niveles altos de azúcar en sangre.
- Latido cardiaco rápido e irregular (fibrilación auricular).
- Pérdida de apetito.
- Estreñimiento, diarrea o náuseas.
- Vómitos.
- Dolor abdominal
- Falta de energía.
- Dificultad para conciliar o mantener el sueño.
- Mareo, temblor.
- Espasmos musculares, debilidad muscular.
- Dolor de huesos, dolor de espalda.
- Entumecimiento, hormigueo o sensación de escozor en la piel, dolor de manos o pies (neuropatía sensitiva periférica).
- Hinchazón generalizada que incluye hinchazón de brazos y piernas.
- Erupciones cutáneas.
- Infección de las vías urinarias, que puede causar una sensación de escozor al orinar o la necesidad de orinar con más frecuencia.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Caída.
- Sangrado en el interior del cráneo.
- Menor capacidad para mover o sentir (sensibilidad) en las manos, pies y piernas debido a un daño neurológico (neuropatía sensitivomotora periférica).
- Entumecimiento, picor u hormigueo en la piel (parestesia).
- Sensación de que la cabeza le da vueltas, lo que le dificulta estar de pie y moverse con normalidad.
- Hinchazón causada por retención de líquidos.
- Habones (urticaria).
- Picor en la piel.
- Herpes zóster.
- Ataque al corazón (dolor de pecho que se extiende a los brazos, el cuello y la mandíbula, sensación de sudoración y dificultad respiratoria, sensación de náuseas o vómitos).
- Dolor torácico, infección en el pecho.
- Aumento de la presión arterial.
- Una reducción del número de glóbulos rojos y blancos y de las plaquetas al mismo tiempo (pancitopenia) que le hará más propenso a las hemorragias y a los moratones. Puede sentirse cansado y débil, así como tener dificultades para respirar. Tendrá también una mayor predisposición a coger infecciones.
- Disminución del número de linfocitos (un tipo de glóbulos blancos) causada a menudo por una infección (linfopenia).
- Niveles bajos de magnesio en sangre (hipomagnesemia), que pueden producir cansancio, debilidad generalizada, calambres musculares e irritabilidad y que pueden producir niveles bajos
- de calcio en sangre (hipocalcemia), lo que puede causar entumecimiento u hormigueo en las manos, pies o labios, calambres musculares, debilidad muscular, aturdimiento, confusión.
- Niveles bajos de fosfato en sangre (hipofosfatemia), que pueden producir debilidad muscular, irritabilidad o confusión.
- Niveles altos de calcio en sangre (hipercalcemia), que pueden ralentizar los reflejos y producir debilidad de los músculos esqueléticos.
- Niveles altos de potasio en sangre, que pueden producir un ritmo cardiaco anormal.
- Niveles bajos de sodio en sangre, que pueden producir cansancio y confusión, contracciones musculares, ataques (convulsiones epilépticas) o coma.
- Niveles altos de ácido úrico en sangre, que pueden producir un tipo de artritis llamado gota.
- Presión arterial baja, que puede causar mareo o desmayo.
- Dolor o sequedad en la boca.
- Cambios en el sabor de las cosas.
- Abdomen hinchado.
- Confusión.
- Sentirse decaído (ánimo depresivo).
- Pérdida de la conciencia, desmayo.
- Opacidad en el ojo (catarata).
- Daño en los riñones.
- Incapacidad para orinar.
- Resultados anómalos en las pruebas de la función hepática.
- Dolor en la pelvis.
- Pérdida de peso.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Ictus.
- Inflamación del hígado (hepatitis) que puede producir picor en la piel, coloración amarillenta en la piel y en la parte blanca de los ojos (ictericia), heces de color claro, orina de color oscuro y dolor abdominal.
- La degradación de las células tumorales tiene como resultado la liberación de compuestos tóxicos en el torrente sanguíneo (síndrome de lisis tumoral). Puede derivar en problemas renales.
- Glándula tiroidea poco activa, lo que puede causar síntomas tales como cansancio, letargo, debilidad muscular, frecuencia cardiaca lenta y aumento de peso.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- Rechazo de trasplante de órganos sólidos (tales como corazón o hígado).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Pomalidomida Teva
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el blíster y la caja después de EXP. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
No utilice Pomalidomida Teva si observa indicios visibles de deterioro o signos de manipulación indebida del medicamento.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Pomalidomida Teva
- El principio activo es pomalidomida
- Los demás componentes son lactosa monohidrato, crospovidona, povidona, laurilsulfato de sodio y estearil fumarato de sodio
Pomalidomida Teva 1 mg cápsulas duras
- Cada cápsula contiene 1 mg de pomalidomida
- La cubierta de la cápsula contiene: azul brillante FCF (E133), dióxido de titanio (E171), gelatina y óxido de hierro amarillo (E172)
- La tinta de impresión contiene: goma laca shellac (E904), propilenglicol (E1520), solución de amoníaco, concentrada (E527), óxido de hierro negro (E172) e hidróxido de potasio (E525)
Pomalidomida Teva 2 mg cápsulas duras
- Cada cápsula contiene 2 mg de pomalidomida
- La cubierta de la cápsula contiene: azul brillante FCF (E133), dióxido de titanio (E171), gelatina, óxido de hierro amarillo (E172) y óxido de hierro rojo (E172)
- La tinta de impresión contiene: goma laca shellac (E904), propilenglicol (E1520), solución de amoníaco, concentrada (E527), óxido de hierro negro (E172) e hidróxido de potasio (E525)
Pomalidomida Teva 3 mg cápsulas duras
- Cada cápsula contiene 3 mg de pomalidomida
- La cubierta de la cápsula contiene: azul brillante FCF (E133), dióxido de titanio (E171), gelatina y óxido de hierro amarillo (E172)
- La tinta de impresión contiene: goma laca shellac (E904), propilenglicol (E1520), solución de amoníaco concentrada (E527), óxido de hierro negro (E172) e hidróxido de potasio (E525)
Pomalidomida Teva 4 mg cápsulas duras
- Cada cápsula contiene 4 mg de pomalidomida
- La cubierta de la cápsula contiene: azul brillante FCF (E133), dióxido de titanio (E171) y gelatina
- La tinta de impresión contiene: goma laca shellac (E904), propilenglicol (E1520), solución de amoníaco, concentrada (E527), óxido de hierro negro (E172) e hidróxido de potasio (E525)
Aspecto del producto y contenido del envase
Pomalidomida Teva 1 mg cápsulas duras: cápsula dura de gelatina de aproximadamente 14 mm con tapa opaca de color azul y cuerpo opaco de color amarillo con la inscripción “T” en la tapa y “1” en el cuerpo.
Pomalidomida Teva 2 mg cápsulas duras: cápsula dura de gelatina de aproximadamente 18 mm con tapa opaca de color azul y cuerpo opaco de color naranja con la inscripción “T” en la tapa y “2” en el cuerpo.
Pomalidomida Teva 3 mg cápsulas duras: cápsula dura de gelatina de aproximadamente 18 mm con tapa opaca de color azul y cuerpo opaco de color verde con la inscripción “T” en la tapa y “3” en el cuerpo.
Pomalidomida Teva 4 mg cápsulas duras: cápsula dura de gelatina de aproximadamente 18 mm con tapa opaca de color azul y cuerpo opaco de color azul claro con la inscripción “T” en la tapa y “4” en el cuerpo.
Cada envase contiene 14, 14 x 1, 21, 21 x 1, 63 y 63 x 1 cápsulas. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
TEVA GmbH
Graf-Arco-Str. 3
89079 Ulm
Alemania
Responsable de la fabricación
Balkanpharma-Dupnitsa AD
3 Samokovsko Shosse Str.
Dupnitsa 2600, Bulgaria
Merckle GmbH
Graf-Arco-Str. 3
89079 Ulm, Alemania
Actavis Group PTC ehf.
Dalshraun 1
IS-220 Hafnarfjordur, Islandia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A. /AG Tel/Tél: +32 3 820 73 73 | Lietuva UAB Teva Baltics Tel: +370 5 266 02 03 |
| Luxembourg/Luxemburg ratiopharm GmbH Tél: +49 731 402 02 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Magyarország Teva Gyógyszergyár Zrt. Tel: (+36) 1 288 6400 |
Danmark Teva Denmark A/S Tlf.: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland Tel: +44 (0) 207 540 7117 |
Deutschland ratiopharm GmbH +49 (0) 731 402 02 | Nederland Teva Nederland B.V. Tel: +31 800 0228 400 |
Eesti UAB Teva Baltics Eesti filiaal Tel.: +372 6610801 | Norge Teva Norway AS Tlf: +47 66 77 55 90 |
| Österreich Ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tel.: + 34 91 387 32 80 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel.: +48 22 345 93 00 |
France Teva Santé Tél: +33 1 55 91 78 00 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda Tel: +351 21 476 75 50 |
Hrvatska Pliva Hrvatska d.o.o Tel: + 385 1 37 20 000 | România Teva Pharmaceuticals S.R.L Tel: +40 21 230 65 24 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 (0) 207 540 7117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Ísland Teva Pharma Iceland ehf. Sími: + 354 550 3300 | Slovenská republika TEVA Pharmaceuticals Slovakia s.r.o Telephone: +421257267911 |
Italia Teva Italia S.r.l Tel:. +39 028917981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 20 180 5900 |
| Sverige Teva Sweden AB Tel: +46 (0)42 12 11 00 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67 323 666 | United Kingdom (Northern Ireland) United Kingdom (Northern Ireland) Teva Pharmaceuticals Ireland Ireland Tel: +44 (0) 207 540 7117 |
Fecha de la última revisión de este prospecto:
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a POMALIDOMIDA TEVA 4 MG CAPSULAS DURAS EFGForma farmacéutica: CAPSULA, 2 mgPrincipio activo: PomalidomidaFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: CAPSULA, 3mgPrincipio activo: PomalidomidaFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: CAPSULA, 4mgPrincipio activo: PomalidomidaFabricante: Bristol-Myers Squibb Pharma EeigRequiere receta
Médicos online para POMALIDOMIDA TEVA 4 MG CAPSULAS DURAS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de POMALIDOMIDA TEVA 4 MG CAPSULAS DURAS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes









