NPLATE 250 microgramos POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar NPLATE 250 microgramos POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Nplate 250 microgramos polvo y disolvente para solución inyectable
Nplate 500 microgramos polvo y disolvente para solución inyectable
romiplostim
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Nplate y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nplate
- Cómo usar Nplate
- Posibles efectos adversos
- Conservación de Nplate
- Contenido del envase e información adicional
- Instrucciones para preparar y administrar una inyección de Nplate
1. Qué es Nplate y para qué se utiliza
El principio activo de Nplate es romiplostim, que es una proteína utilizada para tratar los recuentos bajos de plaquetas en pacientes con trombocitopenia primaria inmune (siglas PTI). La PTI es una enfermedad en la que el sistema inmunitario de su cuerpo destruye sus propias plaquetas. Las plaquetas son las células de la sangre que ayudan a cicatrizar las heridas y forman coágulos de sangre. Los recuentos de plaquetas muy bajos pueden causar hematomas y hemorragias graves.
Nplate se utiliza en pacientes adultos (18 años o mayores) con PTI a los que se les podría o no haber extirpado el bazo y que han sido previamente tratados con corticosteroides o inmunoglobulinas que no les han funcionado.
Nplate funciona estimulando la médula ósea (parte del hueso que genera las células sanguíneas) para que produzca más plaquetas. Esto debería ayudar a evitar los hematomas y las hemorragias relacionadas con la PTI.
2. Qué necesita saber antes de empezar a usar Nplate
No use Nplate
- si es alérgico a romiplostim o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico a otros medicamentos producidos mediante tecnología del ADN que utiliza el microorganismo Escherichia coli(E. coli).
Advertencias y precauciones
- Si deja de tomar Nplate es probable que vuelva a tener un recuento bajo de plaquetas (trombocitopenia). Si deja de tomar Nplate, su recuento de plaquetas tendrá que ser controlado, y su médico le comentará las precauciones adecuadas.
- Si tiene riesgo de aparición de coágulos en la sangre o si los coágulos sanguíneos son frecuentes en su familia. El riesgo de coágulos en la sangre puede estar también aumentado si:
- tiene problemas en el hígado;
- es usted una persona anciana (≥ 65 años);
- está postrado en la cama;
- tiene cáncer;
- está tomando píldoras anticonceptivas o terapia hormonal sustitutiva;
- se ha sometido recientemente a cirugía o ha padecido de una lesión;
- es obeso (sobrepeso);
- es fumador.
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Nplate.
Si presenta un recuento de plaquetas muy alto, puede aumentar el riesgo de coágulos sanguíneos. Su médico ajustará su dosis de Nplate para asegurarse de que su recuento de plaquetas no sea demasiado alto.
Cambios en la médula ósea (aumento de reticulina y posible fibrosis en la médula ósea)
El uso a largo plazo de Nplate puede causar cambios en su médula ósea. Estos cambios pueden llevar a que tenga las células de la sangre anormales o que su cuerpo produzca menor número de células sanguíneas. La forma leve de estos cambios en la médula ósea se llama “aumento de reticulina” y se ha observado en los ensayos clínicos de Nplate. Se desconoce si esto podría progresar a una forma más grave llamada “fibrosis”. En sus análisis de sangre pueden aparecer señales de cambios en la médula ósea como anomalías. Su médico decidirá si un análisis anormal de sangre significa que se debería hacer un análisis de la médula ósea o si debe interrumpir el tratamiento con Nplate.
Empeoramiento del cáncer de la sangre
Su médico puede decidir realizar una biopsia de la médula ósea si considera que es necesario asegurarse que tiene PTI y no otra enfermedad tal como Síndrome Mielodisplásico (SMD). Si usted padece SMD y recibe Nplate, puede presentar un aumento del recuento de células blásticas y un empeoramiento de SMD hasta progresar a una leucemia mieloide aguda, que es un tipo de cáncer de la sangre.
Pérdida de respuesta a romiplostim
Si presenta pérdida de respuesta a romiplostim o imposibilidad de mantener una respuesta a las plaquetas durante el tratamiento con romiplostim, su médico averiguará las razones del porqué, incluyendo si está presentando aumento en las fibras de médula ósea (reticulina) o si ha desarrollado anticuerpos, que neutralizan la actividad de romiplostim.
Niños y adolescentes
No se recomienda el uso de Nplate en niños menores de 18 años.
Otros medicamentos y Nplate
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Si está también tomando otros medicamentos que evitan la formación de coágulos sanguíneos (tratamiento con anticoagulantes o antiplaquetarios) existe un mayor riesgo de hemorragias. Su médico le comentará este aspecto.
Si está tomando corticosteroides, danazol y/o azatioprina, que los podría estar recibiendo para tratar su PTI, puede que haya que reducir o suprimir su administración al combinarlos con Nplate.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No se recomienda utilizar Nplate durante el embarazo a menos que su médico se lo indique.
Se desconoce si romiplostim se excreta en la leche humana. No se recomienda utilizar Nplate durante la lactancia. La decisión de interrumpir la lactancia o interrumpir el tratamiento con romiplostim debe tomarse teniendo en cuenta las ventajas de la lactancia para el bebé y las ventajas del tratamiento con romiplostim para la paciente.
Conducción y uso de máquinas
Consulte a su médico antes de conducir o utilizar máquinas, ya que algunos de los efectos adversos (ej: episodios temporales de mareos) pueden alterar su capacidad para llevar a cabo estas actividades de forma segura.
3. Cómo usar Nplate
Nplate debe administrarse bajo la supervisión directa de un médico que controle con precisión la cantidad de Nplate administrada.
Nplate se administra una vez a la semana mediante inyección debajo de la piel (subcutánea).
La dosis inicial es de 1 microgramo de Nplate por kilogramo de peso corporal una vez por semana. Su médico le indicará la cantidad de Nplate que debe utilizar. Nplate debe inyectarse una vez a la semana para el mantenimiento de los recuentos de plaquetas. Su médico le extraerá muestras de sangre regularmente para evaluar de qué manera están respondiendo sus plaquetas y ajustar la dosis si fuera necesario.
Una vez que su recuento de plaquetas esté controlado, su médico seguirá realizándole análisis de sangre de control. Su dosis puede ajustarse más adelante a fin de mantener un control a largo plazo de su recuento de plaquetas.
Siga siempre exactamente las instrucciones de administración de Nplate indicadas por su médico. En caso de duda, consulte de nuevo a su médico cómo usar Nplate.
Instrucciones para preparar y administrar una inyección de Nplate
Después de una formación adecuada, su médico también le puede permitir autoinyectarse Nplate. Por favor, lea las instrucciones al final de este prospecto sobre cómo inyectar Nplate, tal como ha comentado con su médico. Si su médico le ha permitido autoinyectarse, debe hacer un seguimiento con él todos los meses para que determine si Nplate está funcionando o si debe considerar otro tratamiento.
Tras el primer mes de autoinyectarse Nplate, necesitará demostrar que todavía puede preparar e inyectarse Nplate correctamente.
Si usa más Nplate del que debe
Su médico se asegurará de que usted reciba la cantidad adecuada de Nplate. Si ha recibido más Nplate del que debiera, puede que no presente ningún síntoma físico pero sus niveles de plaquetas en sangre pueden aumentar a niveles muy altos y esto puede aumentar el riesgo de coagulación de la sangre. Por tanto, si su médico sospecha que ha recibido más Nplate del que debiera, se recomienda que sea controlado para observar cualquier signo o síntoma de efectos secundarios y que se le administre el tratamiento adecuado de inmediato.
Si su médico ha permitido que se autoinyecte y utiliza más Nplate del que debería, informe a su médico inmediatamente.
Si usa menos Nplate del que debe
Su médico se asegurará de que usted reciba la cantidad adecuada de Nplate. Si ha recibido menos Nplate del que debe, puede que no presente ningún síntoma físico pero sus niveles de plaquetas en sangre pueden disminuir a niveles bajos y esto puede producir riesgo de sangrado. Por tanto, si su médico sospecha que ha recibido menos Nplate del que debe, se recomienda que sea controlado para observar cualquier signo o síntoma de efectos secundarios y que se le administre el tratamiento adecuado de inmediato.
Si su médico ha permitido que se autoinyecte y utiliza menos Nplate del que debería, informe a su médico inmediatamente.
Si olvidó usar Nplate
Si olvida una dosis de Nplate, su médico le indicará cuándo debe recibir la siguiente dosis.
Si su médico ha permitido que se autoinyecte y olvidó la administración de su inyección, informe a su médico inmediatamente.
Si interrumpe el tratamiento con Nplate
Si deja de utilizar Nplate, es probable que vuelva a presentar un recuento de plaquetas bajo (trombocitopenia). Su médico decidirá si debe dejar de tomar Nplate.
Autoinyectarse Nplate
Su médico puede decidir que es mejor que se inyecte Nplate. Su médico, enfermero o farmacéutico le mostrará cómo se inyecta Nplate. No intente inyectarse usted mismo si no se ha formado. Es muy importante que prepare Nplate correctamente y tome la dosis correcta (ver la sección 7. Instrucciones para la preparación y administración de una inyección de Nplate, al final de este prospecto).
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- dolor de cabeza;
- reacción alérgica;
- infección de la vía respiratoria superior.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- alteración en la médula ósea, incluyendo aumento de las fibras de la médula ósea (reticulina);
- dificultad para conciliar el sueño (insomnio);
- mareos;
- hormigueo o entumecimiento de las manos o los pies (parestesia);
- migraña;
- enrojecimiento de la piel (rubor);
- coágulo de sangre en una arteria del pulmón (embolia pulmonar);
- náuseas;
- diarrea;
- dolor abdominal;
- indigestión (dispepsia);
- estreñimiento;
- picor en la piel (prurito);
- sangrado debajo de la piel (equimosis);
- hematomas (contusión);
- erupción cutánea;
- dolor en la articulación (artralgia);
- dolor muscular o debilidad (mialgia);
- dolor en manos y pies;
- espasmo muscular;
- dolor de espalda;
- dolor de huesos;
- cansancio (fatiga);
- reacción en el lugar de la inyección;
- hinchazón en las manos y los pies (edema periférico);
- síntomas similares a la gripe (enfermedad semejante a la gripe);
- dolor;
- debilidad (astenia);
- fiebre (pirexia);
- escalofríos;
- contusión;
- hinchazón de la cara, labios, boca, lengua o garganta que puede causar dificultad al tragar o respirar (angioedema);
- gastroenteritis;
- palpitaciones;
- inflamación de los senos paranasales (sinusitis);
- inflamación de las vías que transportan el aire hasta los pulmones (bronquitis);
- coagulación sanguínea en las venas (trombosis venosa profunda).
Frecuentes: pueden afectar hasta 1 de cada 10 personas (pueden ser observados en análisis de sangre u orina)
- recuento bajo de plaquetas en sangre (trombocitopenia) y recuento bajo de plaquetas en sangre (trombocitopenia) tras interrumpir el tratamiento con Nplate;
- recuento de plaquetas más elevado de lo normal (trombocitosis);
- anemia.
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- alteración de la medula ósea; trastorno de la medula ósea que provoca cicatrización (mielofibrosis); aumento del tamaño del bazo (esplenomegalia); sangrado de la vagina (hemorragia vaginal), sangrado en el recto (hemorragia rectal); sangrado de la boca (hemorragia bucal); sangrado en el lugar de la inyección (hemorragia en el lugar de la inyección);
- ataque al corazón (infarto de miocardio); aumento de la frecuencia cardíaca;
- mareo o sensación giratoria (vértigo);
- problemas con los ojos incluyendo: sangrado en los ojos (hemorragia conjuntival); dificultad para enfocar o visión borrosa (trastorno de acomodación visual, papiloedema o trastorno de los ojos); ceguera; picor en los ojos (prurito ocular); aumento de lágrimas (aumento del lagrimeo); o alteraciones visuales;
- problemas con el sistema digestivo incluyendo: vómitos; mal aliento (halitosis); dificultad para tragar (disfagia); indigestión o acidez gástrica (trastorno de reflujo gastroesofágico); sangre en las heces (hematoquecia); malestar estomacal; úlceras en la boca o ampollas en la boca (estomatitis); dientes decolorados (decoloración dental);
- pérdida de peso; aumento de peso; intolerancia al alcohol; pérdida de apetito (anorexia o disminución de apetito); deshidratación;
- sensación general de malestar (malestar); dolor en el pecho; irritabilidad; hinchazón de la cara (edema facial); sensación de calor; aumento de la temperatura corporal; sensación nerviosa;
- gripe; infección localizada; inflamación de las fosas de la nariz y la garganta (nasofaringitis);
- problemas en la nariz y la garganta incluyendo: tos; secreción nasal (rinorrea); garganta seca; falta de aliento o dificultad en respirar (disnea); congestión nasal; dolor al respirar (respiración dolorosa);
- articulaciones hinchadas doloridas, causadas por el ácido úrico (producto de degradación de los alimentos) (gota);
- rigidez muscular; debilidad muscular; dolor de hombros; contracciones musculares;
- problemas con el sistema nervioso incluyendo contracciones musculares involuntarias (clonus); sentido del gusto distorsionado (disgeusia); disminución del sentido del gusto (hipogeusia); sensación de disminución de la sensibilidad, especialmente en la piel (hipoaestesia); alteraciones en las funciones nerviosas en brazos y piernas (neuropatía periférica); coágulo sanguíneo en el seno transverso (trombosis de seno transverso);
- depresión; sueños anormales;
- pérdida de cabello (alopecia); sensibilidad a la luz (reacción de fotosensibilidad); acné; reacción alérgica en la piel por el contacto de alérgenos (dermatitis de contacto); manifestaciones de la piel con erupción y ampollas (eczema); piel seca; enrojecimiento de la piel (eritema); descamación grave o erupción descamativa (erupción exfoliativa); crecimiento anormal del pelo; engrosamiento y picor de la piel por rascarse repetidamente (prurigo); sangrado debajo de la superficie de la piel o moretones debajo de la piel (purpura); erupción de la piel con picor (erupción papular); erupción de la piel con picor (erupción pruriginosa); erupción con picor generalizada (urticaria); protuberancia en la piel (nódulo en la piel); olor anormal de la piel (olor anormal de la piel);
- problemas con la circulación incluyendo coágulos sanguíneos en la vena del hígado (trombosis de la vena porta); presión sanguínea baja (hipotensión); aumento de la presión sanguínea; bloqueo de un vaso sanguíneo (embolismo periférico); flujo sanguíneo reducido en las manos, tobillos o pies (isquemia periférica); hinchazón y coagulación en una vena, que puede ser extremamente blanda al tacto (flebitis o tromboflebitis superficial); coagulación sanguínea (trombosis);
- una alteración rara caracterizada por períodos de dolor con quemazón, enrojecimiento y calor en los pies y manos (eritromelalgia).
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas (pueden ser observados en análisis de sangre u orina)
- un tipo raro de anemia en la cual las células rojas de la sangre, las células blancas de la sangre y las plaquetas están reducidas en número (anemia aplásica);
- aumento del recuento de las células blancas de la sangre (leucocitosis);
- exceso en la producción de plaquetas (trombocitaemia); aumento en el recuento de plaquetas; recuento anormal de las células de la sangre que previenen las hemorragias (recuento de plaquetas anormal);
- alteraciones en algunos análisis de la sangre (aumento de las transaminasas; aumento de la lactato deshidrogenasa de la sangre);
- o cáncer de las células blancas de la sangre (mieloma múltiple);
- proteínas en la orina.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nplate
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta del vial después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8°C).
No congelar.
Conservar en el embalaje original para protegerlo de la luz.
Cuando se conserve en el embalaje original, este medicamento puede estar fuera de la nevera durante un período máximo de 30 días a temperatura ambiente (hasta 25°C).
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nplate
- El principio activo es romiplostim.
Cada vial de Nplate 250 microgramos polvo para solución inyectable contiene un total de 375 microgramos de romiplostim. Se ha añadido un volumen adicional en cada vial para asegurar que se pueden administrar 250 microgramos de romiplostim. Tras la disolución, una cantidad de volumen de producto final de 0,5 ml de solución contiene 250 microgramos de romiplostim (500 microgramos/ml).
Cada vial de Nplate 500 microgramos polvo para solución inyectable contiene un total de 625 microgramos de romiplostim. Se ha añadido un volumen adicional en cada vial para asegurar que se pueden administrar 500 microgramos de romiplostim. Tras la disolución, una cantidad de volumen de producto final de 1 ml de solución contiene 500 microgramos de romiplostim (500 microgramos/ml).
- Los demás componentes son:
Polvo: manitol (E421), sacarosa, l-histidina, ácido clorhídrico (para ajuste del pH) y polisorbato 20.
Disolvente: agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Nplate es un polvo blanco para solución inyectable que se suministra en un vial de vidrio de una sola dosis de 5 ml.
Nplate se suministra en un envase o envase múltiple con 4 envases. Cada envase contiene:
1 vial de 250 microgramos o 500 microgramos de romiplostim.
1 jeringa precargada que contiene 0,72 o 1,2 ml de agua para preparaciones inyectables para reconstitución.
1 émbolo para la jeringa precargada.
1 adaptador del vial estéril.
1 jeringa estéril de 1 ml con cierre Luer.
1 aguja de seguridad estéril.
4 toallitas de alcohol.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Países Bajos
Titular de la autorización de comercialización
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Países Bajos
Responsable de la fabricación
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Irlanda
Responsable de la fabricación
Amgen NV
Telecomlaan 5-7
1831 Diegem
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien s.a. Amgen n.v. Tél/Tel: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tél/Tel: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel.: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tlf: +47 23308000 |
| Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
Kúπρος C.A. Papaellinas Ltd Τηλ.: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 |
Fecha de la última revisión de este prospecto en
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu
- Instrucciones para la preparación y administración de una inyección de Nplate
Esta sección contiene información sobre cómo autoinyectarse Nplate. Es importante que no intente autoadministrarse la inyección si no ha recibido formación de su médico, enfermero o farmacéutico. Si usted tiene preguntas acerca de cómo ponerse la inyección, consulte a su médico, enfermero o farmacéutico. Es muy importante que el producto esté preparado correctamente y que la dosis que se administra sea correcta.
Esta sección se divide en las siguientes subsecciones:
Antes de comenzar
Paso 1. Coloque los materiales para la inyección
Paso 2. Prepare el vial para su uso, coloque el adaptador del vial
Paso 3. Prepare la jeringa de agua estéril
Paso 4. Disuelva Nplate inyectando el agua en el vial
Paso 5. Prepare una nueva jeringa para la inyección
Paso 6. Prepare la aguja para la inyección
Paso 7. Elija y prepare la zona de inyección
Paso 8. Inyecte el líquido de Nplate
Paso 9. Eliminación de los suministros
Antes de comenzar
Lea todas las instrucciones de uso cuidadosamente.Estas instrucciones son para pacientes que ya están formados por su profesional sanitario, como su médico, enfermero o farmacéutico, en la autoinyección. Si no ha sido entrenado, por favor póngase en contacto con su profesional sanitario.
El kit de autoinyección de Nplate debe mantenerse en el envase original hasta su utilización con el fin de proteger de la luz el vial de Nplate. Mantener el kit de autoinyección de Nplate refrigerado entre 2°C y 8°C.
Una vez que se ha disuelto Nplate, inyecte inmediatamente.
Es posible que quede un exceso de Nplate después de la administración de la dosis prescrita. ¡No reutilice Nplate! Cualquier exceso de Nplate disuelto, debe ser desechado inmediatamente después de completar el proceso de la inyección. El sobrante de Nplate del vial NUNCA debe ser reutilizado para otra inyección.
Paso 1. Coloque los materiales para la inyección
Realice lo siguiente:
- Seleccione una superficie de trabajo plana y bien iluminada, como una mesa.
- Coja el kit de autoinyección de Nplate de la nevera. No utilizar si está congelado.Si tiene alguna duda sobre la conservación, contacte con su profesional sanitario para más instrucciones.
Verifique la fecha de caducidad incluida en el kit de autoinyección. Si la fecha de caducidad ha pasado, no lo utilice.No continúe y comuníqueselo a su profesional sanitario.
- Nota:Si su médico le ha indicado que la dosis de Nplate requiere más de una inyección, necesitará utilizar más de un kit de autoinyección. Siga los pasos que se describen en este prospecto y utilice tantos kits de autoinyección, como sean necesarios para completar la dosis prescrita de Nplate.
- Asegúrese que tiene los siguientes componentes:
Paquete de toallitas de alcohol x4

1 vial de polvo de 250 microgramos o | Adaptador del vial de 13 mm x1 |
500 microgramos x1 | |

Émbolo para jeringa precargada de agua estéril x1Jeringa precargada de agua estéril x1

Jeringa de 1 ml con cierre Luer x1Aguja de seguridad para la inyección x1

- Noabra los elementos hasta que esté indicado en las instrucciones.
- Noutilice componentes que hayan sido alterados o dañados.
- Noreutilizar los componentes.
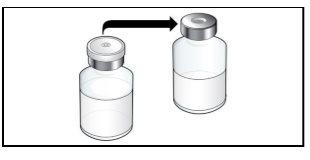
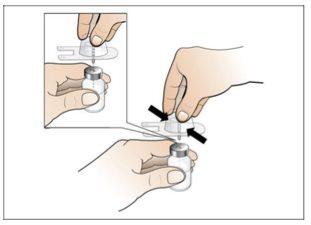
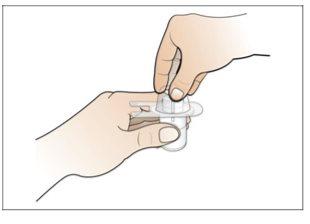
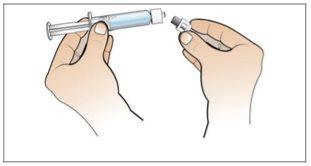
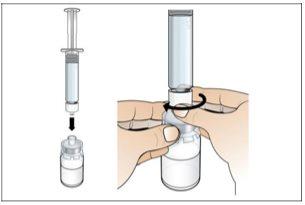
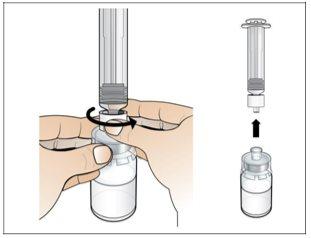
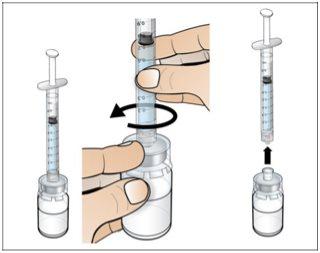
Paso 2. Prepare el vial para su uso, coloque el adaptador del vial
Utilizar:2 paquetes de las toallitas de alcohol, 1 vial y 1 paquete de adaptador del vial.
Realice lo siguiente:
(500 microgramos) del vial. |
|
|
|
|
|
|
|
|
|
|
|
Paso 3. Prepare la jeringa de agua estéril
Utilizar:La jeringa precargada de agua estéril y el émbolo.
Antes de iniciar el Paso 3, por favor tenga en cuenta lo siguiente:
- El plástico transparente del émbolo siempre DEBE estar conectado antes de romper la punta blanca de la jeringa precargada de agua. Lleve a cabo el paso 3a antes del paso 3b.
Realice lo siguiente:
|
|
|
|
|
|
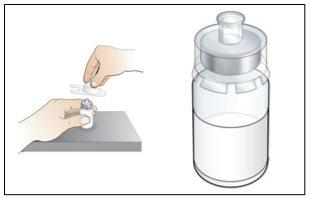
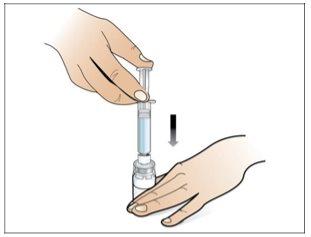
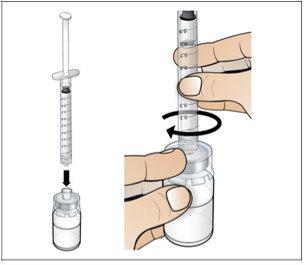
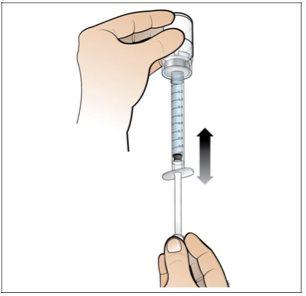
Paso 4. Disuelva Nplate, inyectando el agua en el vial
Utilizar:Jeringa precargada de agua estéril y vial con adaptador de vial adjunto.
Antes de iniciar el Paso 4, por favor tenga en cuenta lo siguiente:
- Disuelvalentamente y con cuidado. Es una proteína y como tal, puede ser fácilmente dañada por agitación de la mezcla inadecuada y excesiva.
Realice lo siguiente:
|
|
|
Presione lenta y suavemente |
Antes de continuar:
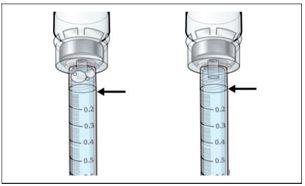
- Asegúreseque toda el agua se inyecta desde la jeringa al vial antes de disolverlo.
|
Correcto |
|
Incorrecto |
Antes de continuar:
- Inspeccionarvisualmente el líquido disuelto en busca de partículas y/o decoloración. Debe ser claro e incoloro y completamente disuelto.
- Nota:Si hay cualquier color o partículas en el líquido,contacte con su profesional sanitario.
- Asegúreseque el líquido está completamente disuelto antes de quitar la jeringa.
|
|
- Deseche la jeringavacía en un contenedor de objetos punzantes o peligrosos. Mantenga el vial disuelto de Nplate. Inmediatamente prepare una nueva jeringa para la inyección.
- Noretarde la inyección de Nplate
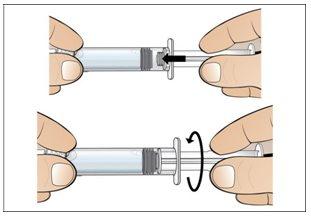
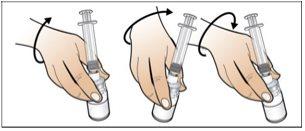
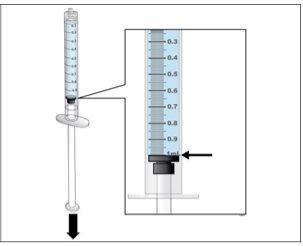
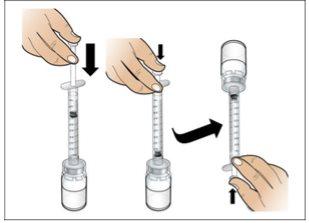
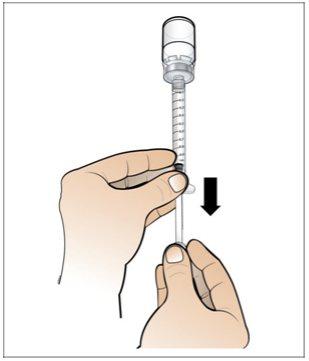
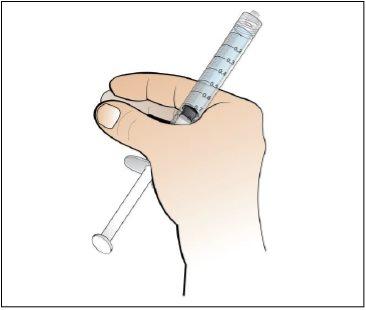
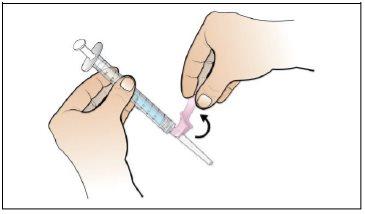
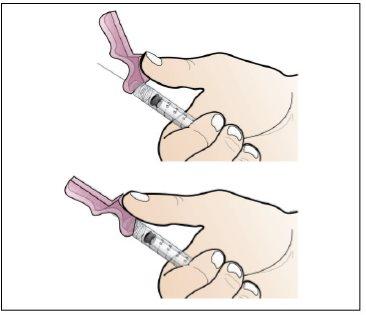
Paso 5. Prepare una nueva jeringa para la inyección
Utilizar:Una nueva jeringa de 1 ml del envase y el vial con Nplate disuelto y trasparente.
Antes de continuar:
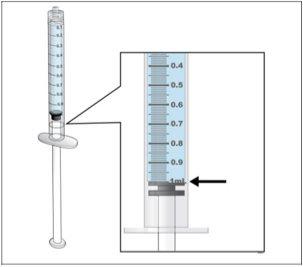
- Revisesu dosis antes de iniciar este paso.
- Nota:Nplate líquido es muy potente, por lo que la exactitud y la medida de la dosis son importantes.
- Asegúreseque se han eliminado todas las burbujas de aire antes de la inyección.
Realice lo siguiente:
|
Ponga aire dentro de la jeringa hasta la marca de 1 ml |
|
|
|
Gire hacia abajo |
|
|
|
Correcto |
|
Burbujas de aire: Correcto Incorrecto |
|
Ajustar la cantidad a su dosis prescrita |
- Haga una comprobación final para asegurar que la cantidad correcta de líquido para su dosis está en la jeringay que todas las burbujas de aire se han retirado.
Antes de continuar:
- Asegúreseque la cantidad correcta de líquido para su dosis se queda en la jeringa.
- Asegúreseque todas las burbujas de aire se retiran de la jeringa.
|
|
|
|
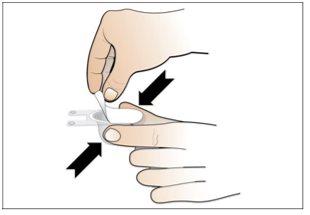
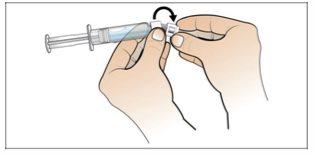
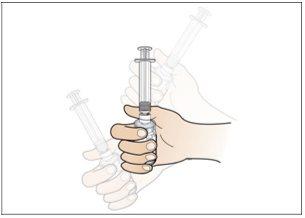
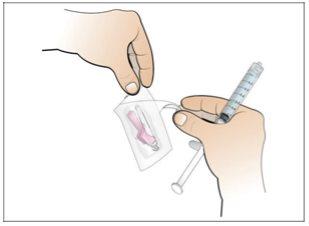
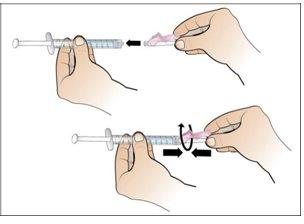
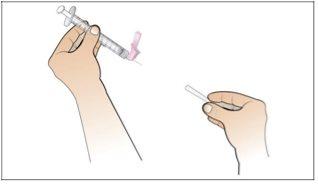
Paso 6. Prepare la aguja para la inyección
Utilizar:La jeringa llena con la dosis medida de Nplate y la aguja de seguridad.
Realice lo siguiente:
|
|
|
|
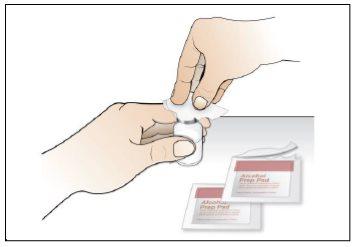
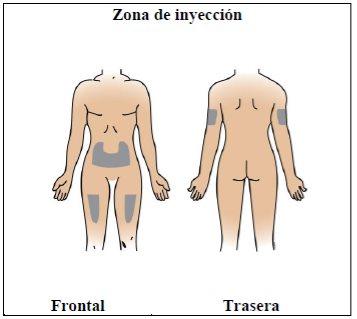
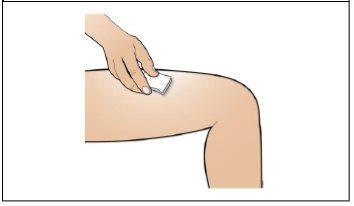
Paso 7. Elija y prepare la zona de inyección
Utilizar:Nuevas toallitas de alcohol.
Realice lo siguiente:
|
|
|
|
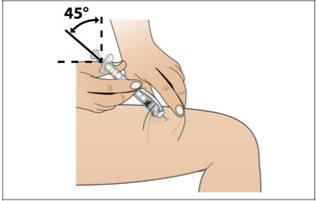
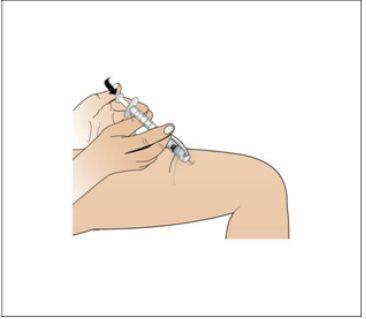
Paso 8. Inyecte el líquido de Nplate
Utilizar:Conjunto con la jeringa llena y la aguja.
Realice lo siguiente:
|
|
|
|
|
|
|
|
|
|
|
|
Paso 9. Eliminación de los suministros
Realice lo siguiente:
- Inmediatamente deseche la jeringa con la aguja cubiertadentro de un contenedor de objetos punzantes.
- Inmediatamente deseche el vial de Nplate utilizadodentro de un contenedor de residuos adecuado.
- Asegúrese que todos los materiales restantes se desechan en los contenedores adecuados.
El dispositivo de inyección y el vial de Nplate NUNCAdeben ser reutilizados.
- Desechela aguja usada y la jeringa en un contenedor resistente a pinchazos.
Desechecualquier resto de Nplate en un contenedor de residuos adecuado. El sobrante de un vial de Nplate NUNCA puede ser reutilizado para otra inyección.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NPLATE 250 microgramos POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 250 µgPrincipio activo: RomiplostimFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 500 µgPrincipio activo: RomiplostimFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 500 µgPrincipio activo: RomiplostimFabricante: Amgen Europe B.V.Requiere receta
Médicos online para NPLATE 250 microgramos POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NPLATE 250 microgramos POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes