NEULASTA 6 mg SOLUCION INYECTABLE

Cómo usar NEULASTA 6 mg SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Neulasta 6 mg solución inyectable
pegfilgrastim
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Neulasta y para qué se utiliza
- Qué necesita saber antes de empezar a usar Neulasta
- Cómo usar Neulasta
- Posibles efectos adversos
- Conservación de Neulasta
- Contenido del envase e información adicional
1. Qué es Neulasta y para qué se utiliza
Neulasta contiene el principio activo pegfilgrastim. Pegfilgrastim es una proteína producida por biotecnología en la bacteria E. coli. Pegfilgrastim pertenece a un grupo de proteínas llamadas citocinas, y es muy similar a una proteína natural (factor estimulador de colonias de granulocitos) producida por nuestro organismo.
Neulasta se usa para reducir la duración de la neutropenia (recuento bajo de glóbulos blancos) y la incidencia de la neutropenia febril (recuento bajo de glóbulos blancos y fiebre) que puede producirse por la quimioterapia citotóxica (medicamentos que destruyen las células que se dividen rápidamente). Los glóbulos blancos son células importantes porque contribuyen a combatir las infecciones. Estas células son sensibles a los efectos de la quimioterapia, lo que puede hacer que su número descienda. Si el número de glóbulos blancos baja mucho, puede que no haya suficientes para combatir las bacterias, lo que implica un riesgo mayor de contraer una infección.
Su médico le ha recetado Neulasta para estimular su médula ósea (la parte del hueso donde se producen las células de la sangre) para que produzca más glóbulos blancos que le ayuden a combatir las infecciones.
2. Qué necesita saber antes de empezar a usar Neulasta
No use Neulasta
- si es alérgico al pegfilgrastim, filgrastim, o a alguno de los demás componentes de este medicamento
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Neulasta:
- si experimenta una reacción alérgica que incluye debilidad, disminución de la presión arterial, dificultad para respirar, hinchazón de la cara (anafilaxis), enrojecimiento y rubor, erupción de la piel y picor en áreas de la piel.
- si tiene alergia al látex. El capuchón de la aguja de la jeringa precargada contiene un derivado del látex que puede causar reacciones alérgicas graves.
- si tiene alergia a los adhesivos acrílicos. El inyector corporal utiliza un adhesivo acrílico que puede causar reacciones alérgicas.
- si experimenta tos, fiebre y dificultad para respirar. Esto puede ser un signo del Síndrome de Distrés Respiratorio Agudo (SDRA).
- si experimenta alguno o una combinación de los siguientes efectos adversos:
? hinchazón que puede estar asociado con orinar con una menor frecuencia, dificultad para respirar, hinchazón y sensación de plenitud abdominal y una sensación general de cansancio.
Estos pueden ser síntomas de una enfermedad llamada “Síndrome de Fuga Capilar” y que puede causar que la sangre se escape de un pequeño vaso sanguíneo hacia otros lugares de su cuerpo. Ver sección 4.
- si tiene dolor en la parte superior izquierda abdominal o dolor en el extremo del hombro. Esto puede ser un signo de un problema con el bazo (esplenomegalia).
- si recientemente tuvo una infección pulmonar grave (neumonía), líquido en los pulmones (edema pulmonar), inflamación de los pulmones (enfermedad intersticial pulmonar) o un resultado anormal de rayos-x del pecho (infiltración pulmonar).
- si es consciente de alguna alteración del recuento de células sanguíneas (por ejemplo, aumento del número de glóbulos blancos o anemia) o una disminución del recuento de plaquetas sanguíneas, que puede reducir la capacidad de la sangre para coagular (trombocitopenia). Su médico puede querer realizarle un mayor seguimiento.
- si tiene anemia de células falciformes. Su médico puede supervisar su enfermedad más estrechamente.
- si es paciente de cáncer de mama o cáncer de pulmón, el tratamiento combinado de Neulasta con quimioterapia y/o radioterapia puede aumentar el riesgo de desarrollar una enfermedad hematológica precancerosa denominada síndrome mielodisplásico (SMD) o una neoplasia hemática denominada leucemia mieloide aguda (LMA). Los síntomas pueden incluir cansancio, fiebre, aparición de cardenales con facilidad o sangrado.
- si tiene signos repentinos de alergia, tales como erupción, picor o urticaria en la piel, hinchazón de la cara, labios, lengua u otras partes del cuerpo, falta de aliento, sibilancias o dificultad para respirar, pueden ser signos de una reacción alérgica grave.
- si tiene síntomas de inflamación de la aorta (el vaso sanguíneo grande que transporta sangre desde el corazón hasta el resto del cuerpo), esto rara vez se ha notificado en pacientes con cáncer y en donantes sanos. Los síntomas pueden incluir fiebre, dolor abdominal, malestar general, dolor de espalda y marcadores inflamatorios aumentados. Informe a su médico si presenta estos síntomas.
Su médico le realizará análisis de sangre y orina de forma regular dado que Neulasta puede dañar los pequeños filtros dentro de los riñones (glomerulonefritis).
Con el uso de Neulasta, se han notificado reacciones cutáneas graves (síndrome de Stevens-Johnson). Deje de usar Neulasta y busque atención médica de inmediato si observa alguno de los síntomas descritos en la sección 4.
Debe consultar con su médico el riesgo de desarrollar cáncer de la sangre. En el caso que desarrolle o pueda desarrollar cáncer de la sangre, no debe utilizar Neulasta, excepto si su médico lo aconseja.
Pérdida de respuesta a pegfilgrastim
Si experimenta una pérdida de respuesta o si no se consigue mantener la respuesta al tratamiento con pegfilgrastim, su médico investigará las causas incluyendo si ha desarrollado anticuerpos que puedan neutralizar la actividad de pegfilgrastim.
Otros medicamentos y Neulasta
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento. Neulasta no se ha usado en mujeres embarazadas. Es importante que informe a su médico si:
- está embarazada;
- cree que pueda estar embarazada; o
- está planeando tener un bebé.
A menos que su médico le indique lo contrario, debe abandonar la lactancia materna si usa Neulasta.
Conducción y uso de máquinas
La influencia de Neulasta sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Neulasta contiene sorbitol (E420) y sodio
Este medicamento contiene 30 mg de sorbitol en cada jeringa precargada equivalente a 50 mg/ml. Este medicamento contiene menos de 1 mmol de sodio (23 mg) por 6 mg de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Neulasta
Neulasta está indicado en pacientes mayores de 18 años.
Siga exactamente las instrucciones de administración de Neulasta indicadas por su médico. Consulte con su médico o farmacéutico si tiene dudas. La dosis habitual es una inyección subcutánea de 6 mg (debajo de la piel), que debe administrarse al final de cada ciclo de quimioterapia a partir de las
24 horas después de su última dosis de quimioterapia.
Autoinyección de Neulasta
Su médico puede considerar más conveniente que se inyecte Neulasta usted mismo. Su médico o enfermero le enseñarán cómo hacerlo. No lo intente si no le han enseñado.
Para más indicaciones sobre cómo inyectarse Neulasta usted mismo, lea la sección 6 al final de este prospecto.
No agite fuertemente Neulasta ya que podría afectar a su actividad.
Si usa más Neulasta del que debe
Si usted usa más Neulasta del que debiera debe informar a su médico, farmacéutico o enfermero.
Si olvidó usar Neulasta
Si usted se está autoinyectando y ha olvidado administrarse su dosis de Neulasta, contacte con su médico para decidir cuándo debe inyectarse la próxima dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe inmediatamente a su médico si experimenta alguno o una combinación de los siguientes efectos adversos:
- hinchazón que puede estar asociado con orinar con una menor frecuencia, dificultad para respirar, hinchazón y sensación de plenitud abdominal y una sensación general de cansancio. Estos síntomas generalmente se desarrollan muy rápidamente.
Estos pueden ser síntomas de una enfermedad que ocurre de forma poco frecuente (que puede afectar hasta 1 de cada 100 personas) llamada “síndrome de fuga capilar” y que puede causar que la sangre se escape de un pequeño vaso sanguíneo hacia otros lugares de su cuerpo y necesite atención médica urgente.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- dolor de huesos. Su médico le informará sobre qué puede tomar para calmar el dolor.
- náuseas y dolor de cabeza.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- con el uso del inyector corporal se han observado erupciones, prurito y urticaria (dermatitis de contacto/reacciones cutáneas locales).
- dolor en la zona de la inyección.
- con el uso del inyector corporal se han observado reacciones en la zona de aplicación que pueden incluir enrojecimiento, sangrado, cardenales, dolor y malestar.
- dolor general y dolor en las articulaciones y músculos.
- pueden tener lugar algunos cambios en su sangre, que serán detectados mediante análisis de sangre periódicos. Puede aumentar el número de glóbulos blancos durante un corto período de tiempo. Puede disminuir el número de plaquetas lo que puede provocar la aparición de moratones.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- reacciones de tipo alérgico, que incluyen enrojecimiento y rubor/sofocos, aparición de sarpullidos, e inflamación cutánea con picor.
- reacciones alérgicas graves, que incluyen anafilaxia (debilidad, caída de la tensión arterial, dificultad para respirar, hinchazón facial).
- aumento del tamaño del bazo.
- ruptura del bazo. Algunos casos de ruptura del bazo fueron mortales. Es importante que contacte con su médico inmediatamente si nota dolor en la parte superior izquierda del abdomen o en el hombro izquierdo ya que podrían tener relación con un problema en su bazo.
- problemas respiratorios. Si usted tiene tos, fiebre y dificultad para respirar, consulte con su médico.
- se han producido casos de síndrome de Sweet (lesiones dolorosas, inflamadas, de coloración violácea en las extremidades y en algunas ocasiones en la cara y cuello, acompañadas de fiebre), pero podrían estar relacionados otros factores.
- vasculitis cutánea (inflamación de los vasos sanguíneos cutáneos).
- daño en los pequeños filtros dentro de los riñones (glomerulonefritis).
- enrojecimientos en la zona de la inyección.
- tos con sangre (hemoptisis).
- trastornos hematológicos (síndrome mielodisplásico [SMD] o leucemia mieloide aguda [LMA]).
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas):
- inflamación de la aorta (el vaso sanguíneo que transporta sangre desde el corazón hasta el resto del cuerpo), ver sección 2.
- sangrado del pulmón (hemorragia pulmonar).
- síndrome de Stevens-Johnson, que puede aparecer como manchas rojizas concéntricas o circulares a menudo con ampollas centrales en el tronco, exfoliación, úlceras en la boca, garganta, nariz, genitales y ojos; y puede venir precedido de fiebre y síntomas tipo gripal. Deje de usar Neulasta si desarrolla estos síntomas y contacte con su médico o busque atención médica de inmediato. Ver sección 2.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Neulasta
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta de la jeringa después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC).
Neulasta puede estar fuera de la nevera a temperatura ambiente (siempre que no supere los 30ºC) durante un máximo de 3 días. Una vez que una jeringa se ha sacado de la nevera y ha alcanzado la temperatura ambiente (que no supere los 30ºC), debe ser utilizada en 3 días o desechada.
No congelar. Neulasta podrá utilizarse en caso de congelación accidental, durante un periodo inferior a 24 horas.
Conservar el envase en el embalaje exterior para protegerlo de la luz.
No utilice este medicamento si observa que la solución no es totalmente transparente o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Neulasta
- El principio activo es pegfilgrastim. Cada jeringa precargada contiene 6 mg de pegfilgrastim en 0,6 ml de solución.
- Los demás componentes son acetato sódico, sorbitol (E420), polisorbato 20 y agua para preparaciones inyectables. Ver sección 2.
Aspecto del producto y contenido del envase
Neulasta es una solución trasparente, incolora inyectable en jeringa precargada (6 mg/0,6 ml).
Cada envase contiene 1 jeringa precargada de vidrio con una aguja de acero inoxidable y un capuchón de la aguja.
La jeringa precargada (envasada con o sin blíster) puede también ser suministrada con un protector automático de la aguja.
Titular de la autorización de comercialización y responsable de la fabricación
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Países Bajos
Titular de la autorización de comercialización
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Países Bajos
Fabricante
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Irlanda
Fabricante
Amgen NV
Telecomlaan 5-7
1831 Diegem
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien s.a. Amgen n.v. Tel/Tél: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tel/Tél: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen, filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tel: +47 23308000 |
| Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 422 0606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
Kúπρος C.A. Papaellinas Ltd Τηλ.: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 | United Kingdom (Northern Ireland) Amgen Limited Tel: +44 (0)1223 420305 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu/.
Instrucciones para inyectarse Neulasta en jeringa precargada
Esta sección contiene información sobre cómo auto-inyectarse Neulasta. Es importante que no intente inyectarse usted mismo si no ha recibido formación específica sobre cómo hacerlo por su médico, enfermero o farmacéutico. Si tiene alguna duda sobre cómo ponerse la inyección, pregunte a su médico, enfermero o farmacéutico.
¿Cómo debe usted o la persona que le va a inyectar, utilizar Neulasta jeringa precargada?
Se tendrá que poner la inyección en el tejido de debajo de la piel. Es lo que se llama una inyección subcutánea.
Equipo necesario para la administración
Para ponerse una inyección subcutánea, necesitará:
- una jeringa precargada de Neulasta; y
- algodón con alcohol o similar.
¿Qué debe hacer antes de ponerse una inyección subcutánea de Neulasta?
- Saque la jeringa precargada de la nevera.
- No agite la jeringa precargada.
- Noretire el capuchón de la jeringa hasta que esté preparado para la inyección.
- Compruebe la fecha de caducidad (EXP) en la etiqueta de la jeringa precargada. No la use si ha pasado el último día del mes indicado.
- Compruebe el aspecto de Neulasta. Debe ser un líquido transparente e incoloro. No lo use si ve partículas.
- Para que la inyección sea más cómoda, deje la jeringa precargada a temperatura ambiente durante 30 minutos o póngasela en la mano cerrada unos minutos. Nocaliente Neulasta de ninguna otra forma (por ejemplo no lo ponga en el microondas, ni en agua caliente).
- Lávese las manos cuidadosamente.
- Busque un lugar cómodo y bien iluminado y coloque todo lo que precise a su alcance.
¿Cómo preparar la inyección de Neulasta?
Antes de inyectarse Neulasta, debe hacer lo siguiente:
|
|
- Puede haber una pequeña burbuja de aire en la jeringa precargada. No es necesario eliminarla antes de la inyección. La inyección de la solución con una burbuja de aire no es perjudicial.
- Ahora ya puede usar la jeringa precargada.
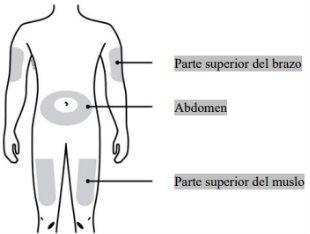
¿Dónde debería ponerse la inyección?
| Los lugares más adecuados para ponerse la inyección uno mismo son:
Si la inyección se la pone otra persona, también se la puede poner en la parte posterior de los brazos. |
¿Cómo ponerse la inyección?
- Limpie la piel usando un algodón con alcohol.
- Pellizque (sin apretar) la piel usando el pulgar y el índice. Inserte la aguja en la piel.
- Presione la cabeza del émbolo con una ligera presión constante. Presione la cabeza del émbolo hasta que se inyecte todo el líquido de la jeringa.
- Tras inyectar la solución, retire la aguja y suelte la piel.
- Si observa un resto de sangre en el lugar de inyección, retírelo con un algodón o gasa. No frote el lugar de inyección. Si es necesario, puede cubrir el lugar de inyección con una tirita.
- No use el resto de Neulasta que quede en la jeringa.
Recuerde
Utilice cada jeringa para una sola inyección. Si tiene algún problema, no dude en pedir ayuda y consejo a su médico o enfermero.
Deshacerse de las jeringas usadas
- No vuelva a poner el capuchón en las agujas ya usadas.
- Mantenga las jeringas usadas fuera de la vista y del alcance de los niños.
- Las jeringas usadas deben eliminarse según la normativa local. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
Instrucciones de uso | |
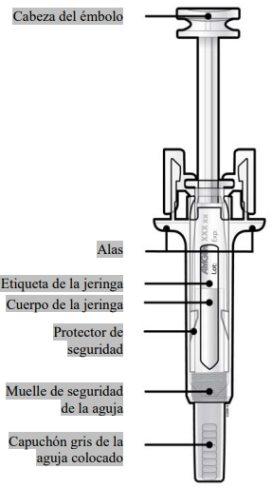
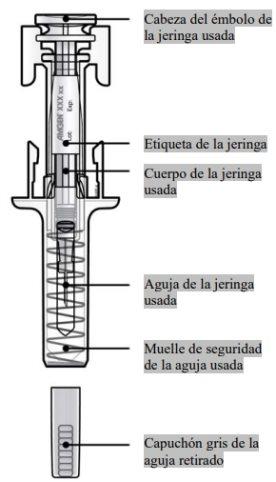
Guía de los componentes | |
Antes de usar | Después de usar |
|
|
Importante |
Lea esta información importante antes de usar la jeringa precargada de Neulasta con protector automático de la aguja:
Noretire el capuchón gris de la aguja de la jeringa precargada hasta que esté preparado para la inyección. Noutilice la jeringa precargada si se ha caído sobre una superficie dura. Utilice una jeringa precargada nueva y contacte con su médico o profesional sanitario. Nointente activar la jeringa precargada antes de la inyección. Nointente quitar el protector de seguridad trasparente de la jeringa precargada. Nointente quitar la etiqueta de la jeringa del cuerpo de la jeringa precargada antes de administrarse la inyección. Si tiene dudas contacte con su médico o profesional sanitario. |
Paso1: Preparación | |
A | Retire el envase de la jeringa precargada que hay en el interior del cartonaje y coja los materiales que necesite para su inyección: toallitas de alcohol, algodón o gasas, tiritas y un contenedor de objetos punzantes (no incluido). |
Para una inyección menos molesta, deje la jeringa precargada a temperatura ambiente durante aproximadamente 30 minutos antes de la inyección. Lávese las manos cuidadosamente con agua y jabón. Coloque la jeringa precargada nueva y los otros materiales sobre una superficie limpia y bien iluminada. Nointente calentar la jeringa utilizando una fuente de calor como el agua caliente o el microondas. Nodeje la jeringa precargada expuesta a la luz solar directa. Noagite la jeringa precargada.
|
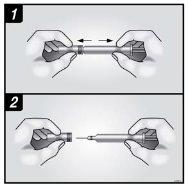
B | Abra el envase, retirando la cubierta. Coja la jeringa precargada por el protector de seguridad para sacarla de la bandeja. |
Coja por aquí Por motivos de seguridad: Nola coja por la cabeza del émbolo. Nola coja por el capuchón gris de la aguja. |
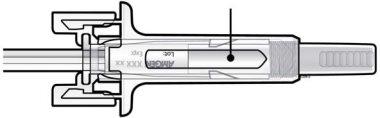
C | Examine el medicamento y la jeringa precargada. |
Medicamento
| |
Noutilice la jeringa precargada si:
En cualquiera de estos casos, contacte con su médico o profesional sanitario. |
Paso 2: Prepárese | |
A | Lávese las manos cuidadosamente. Prepare y limpie el lugar de la inyección. |
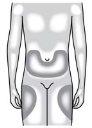
Puede inyectar el medicamento en: La parte superior del muslo. El abdomen, excepto en un área de 5 cm (2 pulgadas) alrededor del ombligo. La cara externa de la parte superior del brazo (solo si la inyección se la administra otra persona). Limpie el lugar de la inyección con una gasa con alcohol. Deje que la piel se seque. Notoque el lugar de la inyección antes de inyectarse | |
Nose inyecte en áreas donde la piel esté sensible, contusionada, enrojecida o con durezas. Evite inyectarse en áreas con cicatrices o estrías. |
B | Tire cuidadosamente del capuchón gris de la aguja en línea recta manteniendo la jeringa separada de su cuerpo. |
|
C | Pellizque el lugar de la inyección para crear una superficie firme. |
| |
|
Paso 3: Inyecte | |
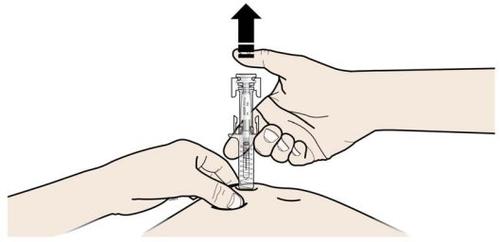
A | Mantenga la piel pellizcada. INSERTE la aguja en la piel. |
Notoque el área limpia de la piel |
B | PRESIONE la cabeza del émbolo con una presión ligera y constante hasta que sienta o escuche un “clic”. Empuje completamente hacia abajo hasta oír el “clic”. |
| |
|
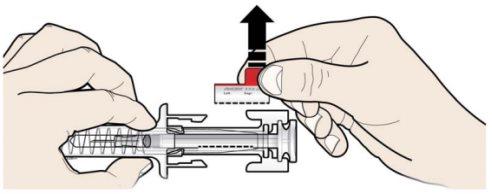
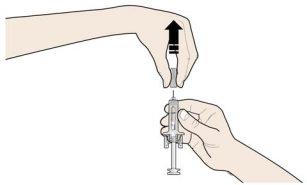
C | DEJE DE PRESIONAR la cabeza del émbolo. A continuación, SEPARE la jeringa de la piel. |
Tras soltar la cabeza del émbolo, el protector de seguridad de la jeringa precargada cubrirá de forma segura la aguja. Novuelva a poner el capuchón gris de la aguja en la jeringa precargada usada. |
Solo para profesionales sanitarios La marca comercial del producto administrado debe estar correctamente registrada en la historia clínica del paciente. |
Retire y guarde la etiqueta de la jeringa precargada.
Gire el émbolo para mover la etiqueta de la jeringa en una posición donde usted pueda retirarla. |
Paso 4: Final | |
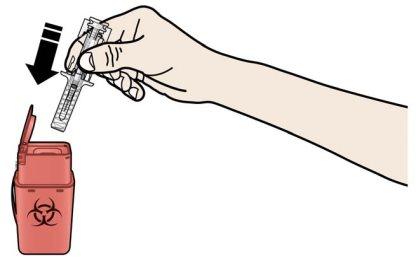
A | Deseche la jeringa precargada usada y otros materiales en un contenedor para desechar objetos punzantes. |
Los medicamentos deben ser eliminados de acuerdo con la normativa local. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente. Mantenga la jeringa y el contenedor de objetos punzantes fuera de la vista y del alcance de los niños. Noreutilice la jeringa precargada. Norecicle las jeringas precargadas ni las tire a la basura. |
B | Examine el lugar de la inyección. |
Si observa sangre, presione con un algodón o una gasa en el lugar de la inyección. No frote en el lugar de la inyección. Si es necesario, ponga una tirita. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NEULASTA 6 mg SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 6 mgPrincipio activo: PegfilgrastimFabricante: Biosimilar Collaborations Ireland LimitedRequiere recetaForma farmacéutica: INYECTABLE, 6 mgPrincipio activo: PegfilgrastimFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 6 mgPrincipio activo: PegfilgrastimFabricante: Pfizer Europe Ma EeigRequiere receta
Médicos online para NEULASTA 6 mg SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NEULASTA 6 mg SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







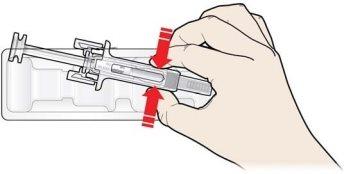

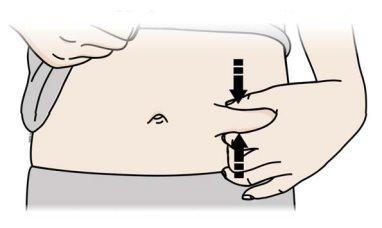
 Es importante mantener la piel pellizcada cuando se inyecte.
Es importante mantener la piel pellizcada cuando se inyecte.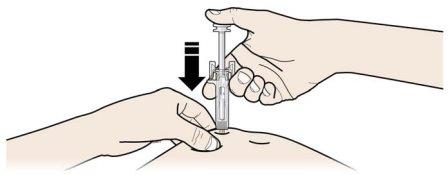
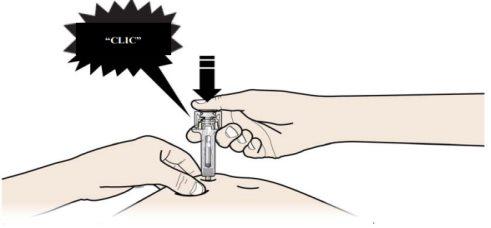
 Es importante presionar hacia abajo hasta oír el “clic” para recibir toda su dosis.
Es importante presionar hacia abajo hasta oír el “clic” para recibir toda su dosis.