
MOVIPREP POLVO PARA SOLUCION ORAL


Cómo usar MOVIPREP POLVO PARA SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Moviprep polvo para solución oral
Macrogol 3350, Sulfato de sodio anhidro, Cloruro de sodio, Cloruro de potasio, Ácido ascórbico y Ascorbato de sodio
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Moviprep y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Moviprep
- Cómo tomar Moviprep
- Posibles efectos adversos
- Conservación de Moviprep
- Contenido del envase e información adicional
1. Qué es Moviprep y para qué se utiliza
Moviprep es un laxante con sabor a limón que se encuentra contenido en cuatro sobres. Hay dos sobres grandes (“sobre A”) y dos sobres pequeños (“sobre B”). Necesita la totalidad de ellos para un tratamiento.
Moviprep está indicado en adultos para limpiar el intestino de modo que esté listo para una exploración. Moviprep actúa vaciando el contenido de su intestino, de modo que usted debe esperar sentir movimientos de evacuación en el mismo.
2. Qué necesita saber antes de empezar a tomar Moviprep
No tome Moviprep:
- si usted es alérgico a los principios activos o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6);
- si usted tiene una obstrucción en el intestino;
- si usted tiene una perforación en la pared de su tracto gastrointestinal;
- si usted tiene alteraciones del vaciamiento gástrico;
- si usted tiene parálisis del tracto gastrointestinal (a veces ocurre tras una operación en el abdomen);
- si usted sufre de fenilcetonuria. Esto es una incapacidad hereditaria del organismo para el empleo de un aminoácido en particular. Moviprep contiene una fuente de fenilalanina;
- si su cuerpo es incapaz de producir suficiente glucosa-6-fosfatodeshidrogenasa;
- si usted tiene megacolon tóxico (una complicación severa de una colitis aguda).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Moviprep. Si usted tiene una salud delicada o una condición médica grave, debe ser consciente de los posibles efectos adversos indicados en la sección 4. Si es su caso, contacte con su médico o farmacéutico.
Consulte a su médico o farmacéutico antes de empezar a tomar Moviprep si presenta alguna de las siguientes situaciones:
- tendencia a regurgitar las bebidas ingeridas, comida o ácido desde el estómago, o si tiene dificultades para tragar (ver también Toma de Moviprep con alimentos y bebidas),
- enfermedad renal,
- tiene insuficiencia cardíaca, problemas renales graves o está tomando medicamentos para la presión arterial,
- fallo cardíaco o enfermedades cardíacas incluyendo hipertensión arterial, latidos cardíacos irregulares o palpitaciones,
- enfermedad tiroidea,
- deshidratación,
- episodios agudos o enfermedad intestinal inflamatoria (enfermedad de Crohn o colitis ulcerosa),
- tiene epilepsia o antecedentes de convulsiones.
Moviprep no debería ser administrado a pacientes con alteraciones de la consciencia sin supervisión médica.
Si presenta vómitos (con sangre) seguidos de dolor repentino en el pecho, cuello o abdomen, dificultad para tragar o dificultad para respirar al tomar Moviprep, deje de tomar el medicamento y contacte inmediatamente con su médico.
Si padece un dolor abdominal o una hemorragia rectal repentinos al tomar Moviprep para la preparación intestinal, póngase en contacto con su médico o acuda a un médico de inmediato.
Niños y adolescentes
Moviprep no debe ser empleado en niños de edad inferior a 18 años.
Toma de Moviprep con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Si usted está tomando otros medicamentos por vía oral (por ejemplo, anticonceptivos orales), no debe tomarlos desde una hora antes, durante y hasta una hora después de la toma de Moviprep, ya que éstos pueden ser eliminados del tracto digestivo y no actuar correctamente.
Si está tomando anticonceptivos orales, podría necesitar usar otros métodos anticonceptivos adicionales (p.ej., condón) para evitar el embarazo.
Toma de Moviprep con alimentos y bebidas
No tomar ningún alimento sólido desde que comience a tomar Moviprep hasta después de la exploración médica.
Si usted necesita espesar líquidos para poder tragarlos de forma segura, Moviprep puede neutralizar el efecto del espesante.
Cuando tome Moviprep debe seguir ingiriendo líquidos en abundancia. El contenido líquido de Moviprep no reemplaza su ingesta habitual de líquidos.
Embarazo, lactancia y fertilidad
No hay datos o éstos son limitados relativos al uso de Moviprep durante el embarazo o lactancia y sólo deberá ser empleado en caso de que el médico lo considere esencial. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Moviprep no afecta a la capacidad para conducir o uso de máquinas.
Moviprepcontiene sodio, potasio y aspartamo
Este medicamento contiene 8,4 g de sodio (componente principal de la sal de mesa/para cocinar) en cada tratamiento (un tratamiento consiste en dos litros de Moviprep). Esto equivale al 420% de la ingesta diaria máxima de sodio recomendada para un adulto. Lo que debe tenerse en cuenta en el tratamiento de pacientes con dietas bajas en sal (sodio). Sólo una proporción (hasta 2,6 g por tratamiento) de sodio es absorbida.
Este medicamento contiene 1,1 g de potasio por tratamiento (un tratamiento consiste en dos litros de Moviprep). Deberá tenerse en cuenta en el tratamiento de pacientes con insuficiencia renal o con dietas bajas en potasio.
Este medicamento puede ser perjudicial para personas con fenilcetonuria porque contiene aspartamo que es una fuente de fenilalanina.
3. Cómo tomar Moviprep
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es de dos litros de solución, la cual se prepara como sigue:
Este envase contiene dos bolsas transparentes con un par de sobres cada una: sobre A y sobre B. Cada par de sobres (A y B) debe ser disuelto en agua para preparar un litro de solución. Este envase contiene por lo tanto cantidad suficiente para preparar dos litros de solución Moviprep.
Antes de tomar Moviprep, por favor lea cuidadosamente las siguientes instrucciones. Usted necesita saber:
- Cuándo tomar Moviprep.
- Cómo preparar Moviprep.
- Cómo beber Moviprep.
- Qué debe esperar que ocurra.
Cuándo tomar Moviprep
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico. En caso de duda, pregunte a su médico. Su tratamiento con Moviprep debe haber sido completado antes de que se lleve a cabo el examen.
Este tratamiento se puede tomar ya sea en dosis divididas o en una única dosis, como se describe a continuación:
Para procedimientos realizados cuando se le duerme (utilizando anestesia general):
- Dosis divididas: un litro de Moviprep en la noche anterior y un litro de Moviprep temprano en la misma mañana del día del examen. Asegúrese de que el consumo de Moviprep, así como cualquier otro líquido claro, haya terminado al menos dos horas antes del inicio del examen.
- Dosis única: dos litros en la noche anterior al procedimiento clínico o dos litros la mañana del examen. Asegúrese de que el consumo de Moviprep, así como cualquier otro líquido claro, haya terminado al menos dos horas antes del inicio del examen.
Para procedimientos realizados sin necesidad de dormirle (sin anestesia general):
- Dosis divididas: un litro de Moviprep en la noche anterior y un litro de Moviprep temprano en la misma mañana del día del examen. Asegúrese de que el consumo de Moviprep, así como cualquier otro líquido claro, haya terminado al menos una hora antes del inicio del examen.
- Dosis única: dos litros de Moviprep en la noche anterior al examen o dos litros de Moviprep la mañana del examen. Asegúrese de que el consumo de Moviprep, haya terminado al menos dos horas antes del inicio del examen. Asegúrese que el consumo de cualquier líquido claro haya terminado al menos una hora antes del examen.
Importante: No tomar ningún alimento sólido desde el comienzo de la toma de Moviprep hasta después de la exploración.
Cómo preparar Moviprep
- Abrir una de las bolsas transparentes y extraer los sobres A y B
- Añadir el contenido de AMBOS, sobre A y sobre B, en una jarra de medición con capacidad de un litro.
- Añadir agua en el recipiente hasta un litro y agitar hasta que todo el polvo se haya disuelto y la solución de Moviprep sea clara o ligeramente turbia. Esto puede llevar hasta cinco minutos.
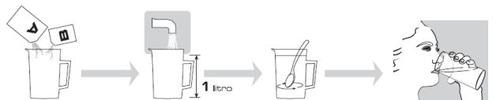
Cómo beber Moviprep
Beba el primer litro de la solución de Moviprep a lo largo de una o dos horas. Intente beber un vaso completo cada 10-15 minutos.
Cuando se encuentre listo, prepare y beba el segundo litro de la solución de Moviprep, preparado con el contenido de los sobres A y B de la bolsa restante.
Durante el curso de este tratamiento, se recomienda que se beba un litro más de un líquido claro, con objeto de prevenir la sensación de encontrarse sediento y prevenir la deshidratación. Agua, caldo, zumos de frutas (sin pulpa), bebidas suaves, té o café (sin leche) son adecuados. Estas bebidas pueden tomarse hasta dos horas antes del examen bajo anestesia general y hasta una hora antes del examen sin anestesia general.
Qué debe esperar usted que ocurra
Cuando comience a beber la solución de Moviprep, es importante que se encuentre cerca de un aseo. En algún momento, comenzará a sentir movimientos intestinales. Esto es completamente normal y ello indica que Moviprep está funcionando. Los movimientos de su intestino cesarán enseguida tras haber terminado de beberlo.
Si sigue estas instrucciones, su intestino quedará limpio, y esto ayudará a que tenga una correcta exploración. Debe reservar el tiempo suficiente para llegar a la unidad de colonoscopia desde la última vez que beba.
Si toma más Moviprep del que debe
Si usted toma más Moviprep del que debe, puede padecer diarrea excesiva que puede dar lugar a deshidratación. Tome abundantes líquidos, especialmente zumos de frutas.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Se recomendará llevar el envase y el prospecto del medicamento al personal sanitario.
Si olvidó tomar Moviprep
Si usted olvida tomar Moviprep, tome la dosis tan pronto como se dé cuenta de que no la ha tomado. Si han transcurrido varias horas desde que tuviera que haberlo tomado, pida consejo a su médico o farmacéutico.
Cuando esté tomando Moviprep como dosis dividida es importante que complete la administración de Moviprep al menos una hora antes del examen (sin anestesia general), o dos horas antes de su examen (con anestesia general). Cuando tome todo el Moviprep en la mañana del examen como dosis única es importante que usted complete la administración de Moviprep al menos dos horas antes del examen.
Si usted tiene más dudas acerca del uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Moviprep puede producir efectos adversos aunque no todas las personas los sufran.
Es normal que aparezca diarrea cuando usted toma Moviprep.
Deje de tomar y dígale a su doctor inmediatamente si usted tiene cualquiera de los siguientes efectos adversos:
- sarpullido o picazón
- hinchazón de la cara, tobillos u otra parte del cuerpo
- palpitaciones
- cansancio extremo
- dificultad para respirar
Estos son síntomas de una reacción alérgica grave.
Deje de tomar Moviprep y contacte inmediatamente con su médico si presenta alguno de los siguientes efectos adversos:
- Convulsiones
Si no tiene movimiento intestinal dentro de las 6 horas tras la toma de Moviprep, detenga el consumo y póngase en contacto con su médico inmediatamente.
Otros efectos adversos incluyen:
- Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes) son: dolor abdominal, distensión abdominal, cansancio, malestar general, irritación en el ano, náuseas y fiebre.
- Efectos adversos frecuentes (pueden afectar hasta a 1 de cada 10 pacientes) son: hambre, alteraciones del sueño, mareo, dolor de cabeza, vómitos, indigestión, sed y escalofríos.
- Efectos adversos poco frecuentes (pueden afectar hasta a 1 de cada 100 pacientes) son: malestar, dificultades para tragar y cambios en las pruebas de función hepática.
Los siguientes efectos adversos han ocurrido alguna vez aunque su frecuencia no es conocida ya que ésta no puede ser estimada con los datos disponibles: flatulencias (gases), incremento temporal de la presión sanguínea, latidos cardíacos irregulares o palpitaciones, deshidratación, arcadas (ganas de vomitar), rotura esofágica provocada por vómito, niveles de sodio sanguíneo muy bajos que pueden causar convulsiones y cambios en los niveles de sales en sangre como disminución de bicarbonato, incremento o disminución del calcio, incremento o disminución del cloruro y disminución del fosfato. Los niveles de potasio y sodio pueden también disminuir particularmente en pacientes que estén tomando medicamentos que afectan a los riñones como inhibidores ECA y diuréticos usados para el tratamiento de enfermedades cardíacas.
Estas reacciones normalmente sólo aparecen mientras dura el tratamiento. Si persisten consulte con su médico.
Reacciones alérgicas que pueden causar una erupción cutánea, picazón, enrojecimiento de la piel o urticaria, inflamación de manos, pies o tobillos, dolores de cabeza, palpitaciones y dificultad para respirar.
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Moviprep
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche después de EXP. Tenga en cuenta que las fechas de caducidad pueden ser diferentes para los distintos sobres. La fecha de caducidad es el último día del mes que se indica.
Conservar los sobres de Moviprep a temperatura ambiente (por debajo de 25°C).
Después de preparar la solución de Moviprep en agua, la solución puede guardarse (manteniéndola cubierta) a temperatura ambiente (por debajo de 25º C). También puede guardarse en nevera (entre 2 y 8º C). No la guarde más de 24 horas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Moviprep
El sobre Acontiene las siguientes sustancias activas:
Macrogol (también conocido como polietilenglicol) 3350 100 g
Sulfato de sodio anhidro 7,500 g
Cloruro de sodio 2,691 g
Cloruro de potasio 1,015 g
El sobre Bcontiene las siguientes sustancias activas:
Ácido ascórbico 4,700 g
Ascorbato de sodio 5,900 g
La concentración de electrolitos cuando ambos sobres se han disuelto en un litro de solución es la siguiente:
Sodio 181,6 mmol/l (de los cuales no más de 56,2 mmol son absorbibles)
Sulfato 52,8 mmol/l
Cloruro 59,8 mmol/l
Potasio 14,2 mmol/l
Ascorbato 56,5 mmol/l
Los demás componentes son:
Aroma de limón (conteniendo maltodextrina, citral, aceite de limón, aceite de lima, goma arábica, vitamina E), aspartamo (E-951) y acesulfamo potásico (E-950) como edulcorantes. Para mayor información consultar la sección 2.
Aspecto del producto y contenido del envase
Este envase contiene dos bolsas transparentes con un par de sobres cada una: sobre A y sobre B. Cada par de sobres (A y B) debe disolverse en un litro de agua.
Moviprep polvo para solución oral se encuentra disponible en estuches de 1, 10, 40, 80, 160 y 320 tratamientos. Envase hospitalario de 40 tratamientos únicos. Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Norgine BV, Antonio Vivaldistraat 150, 1083 HP Ámsterdam, Países Bajos.
Responsable de la fabricación:
Norgine Limited, New Road, Hengoed, Mid Glamorgan, CF82 8SJ, Reino Unido.
o
Norgine BV, Antonio Vivaldistraat 150, 1083 HP Amsterdam, Países Bajos.
o
SOPHARTEX, 21 rue du Pressoir, 28500 Vernouillet, Francia.
o
Recipharm Höganäs AB, Sporthallsvägen 6, Höganäs, 263 35, Suecia.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Norgine de España, S.L.U.
Paseo de la Castellana, 91, 2ª planta
28046, Madrid
España
Este medicamento está autorizado en losestados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) conlos siguientes nombres:
Alemania, Austria, Bélgica, Bulgaria, Dinamarca, Eslovaquia, Eslovenia, España, Estonia, Finlandia, Francia, Irlanda, Islandia, Italia, Letonia, Lituania, Luxemburgo, Malta, Noruega, Países Bajos, Polonia, Portugal, Reino Unido (Irlanda del Norte), República Checa y Rumanía: Moviprep.
Suecia: Movprep.
Fecha de la última revisión de este prospecto:03/2025
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Esta información está destinada únicamente a profesionales del sector sanitario:
Moviprep debe ser administrado con precaución en caso de pacientes delicados con salud pobre o en pacientes con alteraciones clínicas graves tales como:
La presencia de deshidratación o desequilibrios electrolíticos debe ser corregida antes del uso de Moviprep. Pacientes semi-inconscientes o pacientes con riesgo de aspiración o regurgitación, deberán ser cuidadosamente observados durante la administración, especialmente si ésta tiene lugar mediante vía nasogástrica. Moviprep no debe ser administrado a pacientes inconscientes. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MOVIPREP POLVO PARA SOLUCION ORALForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 13125 MG + 350,7 MG + 178,5 MG + 46,6 MGPrincipio activo: macrogol, combinationsFabricante: Lainco S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, gPrincipio activo: macrogol, combinationsFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 13,7 gPrincipio activo: macrogol, combinationsFabricante: Italfarmaco S.A.Requiere receta
Médicos online para MOVIPREP POLVO PARA SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MOVIPREP POLVO PARA SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















