
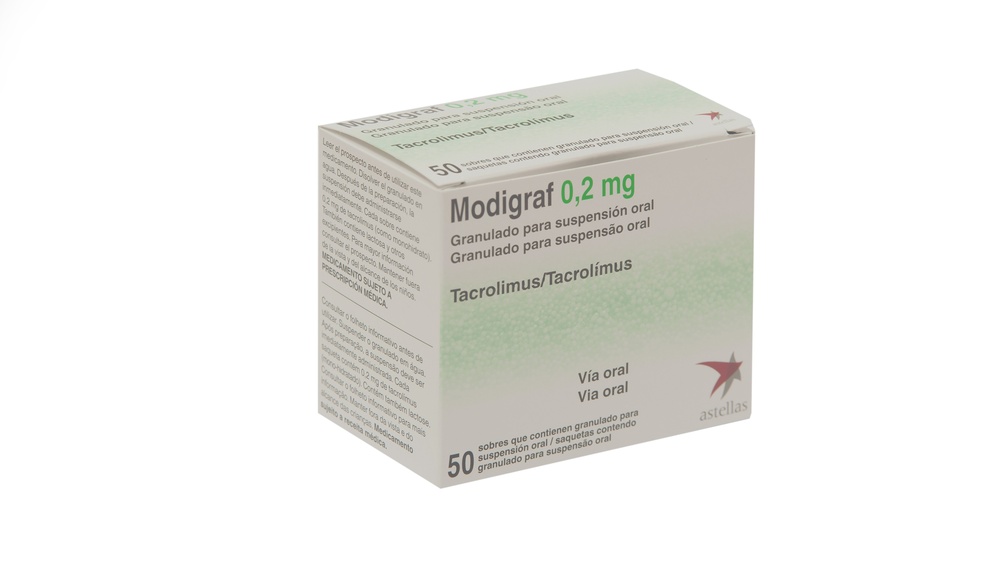
MODIGRAF 0,2 mg GRANULADO PARA SUSPENSION ORAL

Cómo usar MODIGRAF 0,2 mg GRANULADO PARA SUSPENSION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Modigraf 0,2mg, granulado para suspensión oral
Modigraf 1mg, granulado para suspensión oral
Tacrólimus
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Modigraf y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Modigraf
- Cómo tomar Modigraf
- Posibles efectos adversos
- Conservación de Modigraf
- Contenido del envase e información adicional
1. Qué es Modigraf y para qué se utiliza
Modigraf contiene el principio activo tacrólimus. Es un inmunosupresor. Tras su trasplante de órgano (p. ej., hígado, riñón, corazón), el sistema inmunitario de su organismo intentará rechazar el nuevo órgano. Modigraf se utiliza para controlar la respuesta inmune de su cuerpo, permitiéndole aceptar el órgano trasplantado.
También puede recibir Modigraf para tratar un rechazo que se esté produciendo de su hígado, riñón, corazón u otro órgano trasplantado o si cualquier tratamiento previo que estuviera siguiendo no consigue controlar esta respuesta inmunitaria después de su trasplante.
Modigraf se usa en adultos y niños.
2. Qué necesita saber antes de empezar a tomar Modigraf
No tome Modigraf
- Si es alérgico al tacrólimus o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si es alérgico al sirólimus (otra sustancia utilizada para prevenir el rechazo de su órgano trasplantado) o a cualquier antibiótico macrólido (p. ej., eritromicina, claritromicina, josamicina).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Modigraf
- si tiene o ha tenido problemas de hígado
- si ha tenido diarrea durante más de un día
- si siente fuerte dolor abdominal acompañado o no de otros síntomas, como escalofríos, fiebre, náuseas o vómitos
- si tiene una alteración de la actividad eléctrica de su corazón llamada “prolongación del intervalo QT”
- si tiene o ha tenido lesiones en los vasos sanguíneos de menor tamaño, conocidas como microangiopatía trombótica/púrpura trombocitopénica trombótica/síndrome hemolítico urémico. Informe a su médico si desarrolla fiebre, hematomas debajo de la piel (pueden aparecer como puntos rojos), cansancio inexplicable, confusión, coloración amarillenta de la piel o los ojos, descenso en el volumen de orina, pérdida de visión y convulsiones (ver sección 4). Cuando se toma tacrólimus junto con sirólimus o everólimus, el riesgo de que aparezcan estos síntomas puede aumentar.
Por favor evite tomar cualquier preparado a base de plantas, p. ej., la hierba de San Juan (Hypericum perforatum) o cualquier otro producto a base de plantas, ya que esto puede afectar la efectividad y la dosis de Modigraf que necesita recibir. Si tiene alguna duda, por favor consulte a su médico antes de tomar cualquier producto o preparado a base de plantas.
Su médico puede necesitar ajustarle su dosis de Modigraf.
Debe mantenerse en contacto habitual con su médico. De vez en cuando, para establecer la dosis adecuada de Modigraf, es posible que su médico necesite realizarle un análisis de sangre y orina, pruebas cardíacas, pruebas oculares.
Debe limitar su exposición a la luz solar y a la UV (ultravioleta) mientras toma Modigraf. Esto es porque los inmunosupresores como Modigraf pueden aumentar el riesgo de cáncer de piel. En caso de exposición solar, lleve ropa protectora adecuada, y use un protector solar con un factor de protección solar elevado.
Precauciones de manipulación:
Durante la preparación se debe evitar el contacto de cualquier parte del cuerpo como la piel o los ojos, así como respirar cerca de las soluciones para inyección, polvo o granulado contenidos en los productos tacrólimus. Si tal contacto se produce, lave la piel y los ojos.
Otros medicamentos y Modigraf
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se recomienda el uso de Modigraf con ciclosporina (otro medicamento empleado para la prevención del rechazo del trasplante de órganos).
Si necesita visitar a un médico diferente de su especialista en trasplantes, diga al médico que está tomando tacrólimus.Es posible que su médico necesite consultar a su especialista en trasplantes si usted debe usar otro medicamento que pudiera aumentar o reducir su nivel sanguíneo de tacrólimus.
Los niveles sanguíneos de Modigraf pueden modificarse debido a otros medicamentos que esté tomando, y los niveles sanguíneos de otros medicamentos pueden modificarse por la administración de Modigraf, lo que puede requerir la interrupción, aumento o disminución de la dosis de Modigraf.
Algunos pacientes han experimentado aumentos en los niveles sanguíneos de tacrólimus mientras tomaban otros medicamentos. Esto podría provocar efectos adversos graves, tales como problemas de riñón, problemas del sistema nervioso y trastornos del ritmo cardiaco (ver sección 4).
El efecto sobre los niveles sanguíneos de Modigraf se puede producir muy pronto tras empezar a usar otro medicamento, así que puede que sea necesario monitorizar el nivel sanguíneo de Modigraf de manera frecuente y continuada durante los primeros días de uso de otro medicamento y con frecuencia mientras continúe su uso. Algunos otros medicamentos pueden provocar que los niveles sanguíneos de tacrólimus se reduzcan, lo que puede aumentar el riesgo de rechazo del órgano trasplantado. En particular, debe informar a su médico si está tomando o ha tomado recientemente medicamentos como:
- antifúngicos y antibióticos, especialmente los llamados antibióticos macrólidos, empleados para el tratamiento de infecciones, por ej., ketoconazol, fluconazol, itraconazol, posaconazol, voriconazol, clotrimazol, isavuconazol, miconazol, caspofungina, telitromicina, eritromicina, claritromicina, josamicina, azitromicina, rifampicina, rifabutina, isoniazida y flucloxacilina
- letermovir, utilizado para prevenir enfermedades causadas por el CMV (citomegalovirus humano)
- inhibidores de la proteasa del VIH (por ej., ritonavir, nelfinavir, saquinavir), el fármaco potenciador cobicistat y los comprimidos combinados o inhibidores de transcriptasa inversa no nucleósidos para VIH (efavirenz, etravirina, nevirapina) utilizados para tratar infecciones por el VIH
- inhibidores de la proteasa del VHC (por ej., telaprevir, boceprevir, la combinación ombitasvir/paritaprevir/ritonavir, con o sin dasabuvir, elbasvir/grazoprevir y glecaprevir/pibrentasvir), utilizados para tratar la infección por hepatitis C
- nilotinib e imatinib, idelalisib, ceritinib, crizotinib, apalutamida, enzalutamida o mitotano (usados para tratar algunos tipos de cáncer)
- ácido micofenólico, utilizado para suprimir el sistema inmunitario como prevención del rechazo de trasplante
- medicamentos para úlcera de estómago y reflujo ácido (p. ej., omeprazol, lansoprazol o cimetidina)
- antieméticos, utilizados para tratar náuseas y vómitos (p. ej., metoclopramida)
- cisaprida o el antiácido hidróxido de magnesio-aluminio, utilizados para tratar la acidez
- la píldora anticonceptiva, tratamientos hormonales con etinilestradiol o tratamientos hormonales con danazol
- medicamentos empleados para tratar la hipertensión arterial o problemas cardíacos (p. ej., nifedipino, nicardipino, diltiazem y verapamilo)
- medicamentos antiarrítmicos (amiodarona) utilizados para controlar la arritmia (latido irregular del corazón)
- los medicamentos conocidos como “estatinas” empleados para tratar el colesterol y los triglicéridos elevados
- carbamazepina, fenitoína o fenobarbital, empleados para tratar la epilepsia
- metamizol, empleado para tratar el dolor y la fiebre
- los corticosteroides prednisolona o metilprednisolona, pertenecientes a la clase de los corticosteroides utilizados para tratar inflamaciones o suprimir el sistema inmune (por ej., rechazo del trasplante)
- nefazodona, empleada para tratar la depresión
- medicamentos a base de plantas que contengan hierba de San Juan (Hypericum perforatum) o extractos de Schisandra sphenanthera
- cannabidiol (su uso incluye, entre otros, el tratamiento de las crisis epilépticas).
- Informe a su médico si está recibiendo tratamiento para la hepatitis C. El tratamiento farmacológico para la hepatitis C puede hacer que cambie su función hepática y puede afectar a los niveles sanguíneos de tacrólimus. Los niveles sanguíneos de tacrólimus pueden reducirse o aumentar dependiendo de los medicamentos recetados para la hepatitis C. Es posible que su médico necesite monitorizar estrechamente los niveles sanguíneos de tacrólimus y hacer los ajustes necesarios de la dosis de Modigraf después de iniciar el tratamiento para la hepatitis C.
Informe a su médico si está tomando o necesita tomar ibuprofeno (utilizado para tratar la fiebre, la inflamación y el dolor), antibióticos (cotrimoxazol, vancomicina o antibióticos aminoglucósidos como gentamicina), anfotericina B (utilizado para tratar infecciones producidas por hongos) o antivirales (utilizados para tratar infecciones víricas p. ej., aciclovir, ganciclovir, cidofovir, foscarnet). Estos medicamentos pueden empeorar problemas de riñón o del sistema nervioso cuando se toman conjuntamente con Modigraf.
Informe a su médico si está tomando sirólimus o everólimus. Cuando se toma tacrólimus junto con sirólimus o everólimus, el riesgo de que aparezca microangiopatía trombótica, púrpura trombocitopénica trombótica y síndrome hemolítico urémico puede aumentar (ver sección 4).
Su médico también necesita saber si está tomando suplementos de potasio o ciertos diuréticos utilizados para insuficiencia cardíaca, hipertensión y nefropatía, (p. ej., amilorida, triamtereno o espironolactona), o los antibióticos trimetoprima y cotrimoxazol que pueden aumentar los niveles de potasio en su sangre, fármacos antiinflamatorios no esteroideos (AINEs, p. ej., ibuprofeno) utilizados para fiebre, inflamación y dolor, anticoagulantes (que evitan la coagulación de la sangre), o medicamentos orales para el tratamiento de diabetes, mientras toma Modigraf.
Si tiene previsto vacunarse, consulte a su médico.
Toma de Modigraf con alimentos y bebidas
Por lo general, debe tomar Modigraf con el estómago vacío o por lo menos 1 hora antes o de 2 a 3 horas después de una comida. Evite el pomelo o el zumo de pomelo mientras está tomando Modigraf, puesto que puede afectar a sus niveles en la sangre.
Embarazo y lactancia
Si toma Modigraf durante el embarazo, puede que pase a su bebé a través de la placenta. Podría influenciar potencialmente en la salud de su bebé o perjudicar el curso del embarazo.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Un estudio evaluó los resultados del embarazo en mujeres tratadas con tacrólimus y con otros inmunosupresores. Aunque en este estudio no hubo suficiente evidencia para sacar conclusiones, se reportaron tasas más altas de aborto espontáneo entre pacientes con trasplante de hígado y riñón tratadas con tacrólimus, así como tasas más altas de hipertensión persistente, asociada con pérdida de proteínas en la orina, entre pacientes de trasplante de riñón que se desarrolla durante el embarazo o el período posparto (una condición llamada preeclampsia). No se encontró un mayor riesgo de defectos congénitos graves asociados con el uso de Modigraf. Modigraf pasa a la leche materna. Por tanto, no debe dar de mamar mientras tome Modigraf.
Conducción y uso de máquinas
No conduzca y no maneje herramientas o máquinas si se siente mareado o somnoliento, o tiene problemas para ver con claridad después de tomar Modigraf. Estos efectos son más frecuentes si también toma alcohol.
Modigraf contiene lactosa y sodio
Modigraf contiene lactosa (azúcar de la leche). Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por sobre; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Modigraf
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Modigraf lo deben prescribir los médicos con experiencia para tratar pacientes trasplantados y en el uso de medicamentos que controlan el sistema inmune del organismo (inmunosupresores).
Asegúrese de que recibe el mismo medicamento con tacrólimus cada vez que recoja su prescripción médica, a menos que su especialista en trasplante haya acordado cambiar a otro medicamento diferente con tacrólimus.
Este medicamento debe tomarse dos veces al día. Si el aspecto físico ha cambiado del granulado blanco normal, o si las instrucciones de dosis han cambiado, consulte a su médico o farmacéutico lo antes posible para asegurarse de que tiene el medicamento correcto.
La dosis inicial para prevenir el rechazo de su órgano trasplantado será fijada por su médico calculándola según su peso corporal. Las dosis iniciales justo después del trasplante estarán generalmente dentro del intervalo de 0,075 – 0,30 mg por kg de peso corporal y por día, dependiendo del órgano trasplantado. Para el tratamiento del rechazo, se puede emplear la misma dosis.
Su dosis depende de sus condiciones generales, y de otros medicamentos inmunosupresores que pueda estar tomando.
Niños y adolescentes
Los niños y adolescentes recibirán dosis de Modigraf calculadas de la misma forma que las de los adultos. En general, los niños necesitan dosis por kg de peso más altas para alcanzar los mismos niveles eficaces en sangre que los adultos.
Tras el inicio de su tratamiento con Modigraf, su médico le realizará análisis sanguíneos frecuentes para definir la dosis correcta y para ajustar la dosis de vez en cuando. Su médico disminuirá habitualmente su dosis de Modigraf una vez que su situación se haya estabilizado. Su médico le dirá exactamente cuántos sobres debe tomar.
Usted va a necesitar tomar Modigraf todos los días hasta que siga necesitando inmunosupresión para prevenir rechazo de su órgano trasplantado. Debe mantenerse en contacto habitual con su médico.
Modigraf se toma por vía oral dos veces al día, normalmente por la mañana y por la noche. Tome Modigraf con el estómago vacío o de 2 a 3 horas después de una comida. Espere al menos una hora hasta la siguiente comida.
Cómo preparar los sobres de Modigraf para su uso
Su médico le aconsejará sobre el número de sobres que tiene que abrir y el volumen de agua necesaria para hacer la suspensión. Para medir con precisión el volumen de agua puede utilizar una jeringa o un recipiente graduado.
Vierta el volumen prescrito de agua (a temperatura ambiente) en un vaso o taza, hasta un máximo de 50 ml. Coloque la taza con el agua sobre una superficie estable. No utilice tazas ni cucharas hechas de PVC (polivinilcloruro) para preparar Modigraf porque el principio activo del Modigraf puede adherirse al PVC.
Abra cuidadosamente el número de sobres prescritos, por ejemplo, con unas tijeras por el punto indicado con una flecha. Sostenga el sobre abierto entre los dedos pulgar e índice sobre la taza con el lado abierto del sobre hacia abajo. Dé ligeros golpecitos en el extremo cerrado del sobre y vierta el contenido de cada uno de los sobres en el vaso o taza con agua. No utilice ningún utensilio ni líquidos para vaciar el sobre. Si sigue estas instrucciones, obtendrá la cantidad correcta del granulado del sobre. Es normal que quede algo del granulado en el interior; el sobre está diseñado para eso.
Agite o remueva suavemente hasta que el granulado esté en suspensión por completo. La suspensión se puede coger con una jeringa o el paciente lo puede tomar directamente. El líquido tiene un sabor dulce. Enjuague el vaso o la taza una vez con la misma cantidad de agua y bébaselo también. El líquido debe beberse inmediatamente tras su preparación.
Si toma más Modigraf del que debe
Si por accidente toma más Modigraf del que debe, contacte con su médico o acuda al servicio de urgencias del hospital más cercano.
Si olvidó tomar Modigraf
No tome una dosis doble para compensar las dosis individuales olvidadas.
Si se le ha olvidado tomar Modigraf, espere hasta que sea la hora de la dosis siguiente y continúe entonces como antes.
Si interrumpe el tratamiento con Modigraf
La interrupción de su tratamiento con Modigraf puede aumentar el riesgo de rechazo de su órgano trasplantado.
No interrumpa el tratamiento a no ser que su médico se lo diga.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Modigraf reduce los mecanismos de defensa de su cuerpo (sistema inmune), el cual no funcionará tan bien a la hora de luchar contra las infecciones. Por lo tanto, si usted está tomando Modigraf, será más propenso a sufrir infecciones.
Algunas infecciones pueden ser graves o fatales y pueden incluir infecciones provocadas por bacterias, virus, hongos, parásitos u otras infecciones.
Informe a su médico inmediatamente si tiene síntomas de una infección, incluyendo:
- Fiebre, tos, dolor de garganta, sensación de debilidad o malestar general
- Pérdida de memoria, problemas para pensar, dificultad para andar o pérdida de visión – estos síntomas pueden ser debidos a una infección del cerebro muy rara y grave que puede ser fatal (leucoencefalopatía multifocal progresiva o LMP)
Pueden producirse efectos graves, incluidas reacciones alérgicas y anafilácticas (un tipo muy grave de reacción alérgica con lipotimia y dificultad para respirar, que requiere atención médica inmediata). Se han comunicado tumores benignos y malignos después del tratamiento con Modigraf.
Informe a su médico inmediatamente si tiene sospechas de sufrir alguno de los siguientes efectos adversos graves:
Efectos adversos graves frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Perforación gastrointestinal: fuerte dolor abdominal acompañado o no con otros síntomas como pueden ser escalofríos, fiebre, náuseas o vómitos.
- Función insuficiente de su órgano trasplantado.
- Visión borrosa.
Efectos adversos graves poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Microangiopatía trombótica (lesiones en los vasos sanguíneos de menor tamaño) incluido el síndrome hemolítico urémico con los siguientes síntomas: volumen de orina bajo o nulo (fallo renal agudo), cansancio extremo, coloración amarillenta de la piel o los ojos (ictericia) y hematomas o sangrado anormal y signos de infección.
Efectos adversos graves raros (pueden afectar hasta 1 de cada 1.000 personas):
- Púrpura trombocitopénica trombótica: incluye lesiones en los vasos sanguíneos de menor tamaño y se caracteriza por fiebre y hematomas debajo de la piel que pueden aparecer como puntos rojos, con o sin un cansancio extremo inexplicable, confusión, coloración amarillenta de la piel o los ojos (ictericia), con síntomas de fallo renal agudo (volumen de orina bajo o nulo), pérdida de visión y convulsiones.
- Necrólisis epidérmica tóxica: erosión y aparición de ampollas en la piel o en las membranas mucosas, piel enrojecida e hinchada que puede descamarse en amplias partes del cuerpo.
- Ceguera.
Efectos adversos graves muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- Síndrome de Stevens-Johnson: inexplicable dolor de piel generalizado, hinchazón facial, enfermedad grave con formación de ampollas en la piel, boca, ojos y genitales, ronchas, hinchazón de la lengua, erupción cutánea roja o púrpura que se propaga, descamación de la piel.
- Torsades de pointes: cambio en la frecuencia cardiaca que puede venir o no acompañado de síntomas como dolor de pecho (angina de pecho), desmayos, vértigos o náuseas, palpitaciones (sintiendo los latidos cardiacos) y dificultad para respirar.
Efectos adversos graves de frecuencia no conocida (la frecuencia no puede estimarse a partir de los datos disponibles):
- Infecciones oportunistas (bacterianas, fúngicas, víricas y protozoarias): diarrea prolongada, fiebre y dolor de garganta.
- Se han comunicado tumores benignos y malignos tras el tratamiento como resultado de la inmunosupresión, incluyendo cánceres de piel malignos y un tipo de cáncer raro que puede incluir lesiones de la piel conocido como sarcoma de Kaposi. Los síntomas incluyen alteraciones en la piel como nuevas coloraciones o cambios en las existentes, lesiones o bultos.
- Se han comunicado casos de aplasia eritrocitaria pura (una reducción muy considerable del recuento de glóbulos rojos), anemia hemolítica (disminución del número de glóbulos rojos debido a una rotura anormal de éstos acompañado de cansancio) y neutropenia febril (una disminución en el tipo de glóbulos blancos que combaten las infecciones, acompañada de fiebre). No se conoce la frecuencia exacta con la que ocurren estos efectos adversos. Puede no tener síntomas o dependiendo de las condiciones de gravedad usted puede sentir: fatiga, apatía, palidez anormal de la piel (palidez), dificultad para respirar, mareo, dolor de cabeza, dolor de pecho y sensación de frío en manos y pies.
- Casos de agranulocitosis (una disminución considerable en el número de glóbulos blancos acompañado de llagas en la boca, fiebre e infección(es)). Puede no tener síntomas o puede sentir fiebre, escalofríos y dolor de garganta de forma repentina.
- Reacciones alérgicas y anafilácticas con los síntomas siguientes: erupción cutánea con picazón repentina (ronchas), hinchazón de manos, pies, tobillos, cara, labios, boca o garganta (que pueden causar dificultad para tragar o respirar) y puede sentir que se va a desmayar.
- Síndrome de encefalopatía posterior reversible (SEPR): dolor de cabeza, confusión, cambios de estado de ánimo, ataques y alteraciones de la visión. Estos podrían ser signos de un trastorno conocido como síndrome de encefalopatía posterior reversible, que se ha comunicado en algunos pacientes tratados con tacrólimus.
- Neuropatía óptica (alteración del nervio óptico): problemas de visión como visión borrosa, cambios en la visión de los colores, dificultad para ver detalles o reducción del campo visual.
Después de recibir Modigraf también se pueden producir los efectos adversos siguientes y pueden ser graves:
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Aumento del azúcar en la sangre, diabetes mellitus, aumento del potasio en la sangre.
- Dificultad para dormir.
- Temblores, dolor de cabeza.
- Aumento de la presión arterial.
- Anomalías en las pruebas de función del hígado.
- Diarrea, náuseas.
- Problemas renales.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Reducción en el número de células sanguíneas (plaquetas, glóbulos rojos o glóbulos blancos), aumento del recuento de glóbulos blancos, cambios en los recuentos de glóbulos rojos (ver análisis de sangre).
- Reducción del magnesio, el fosfato, el potasio, el calcio o el sodio en la sangre, sobrecarga de líquidos, aumento del ácido úrico o los lípidos en la sangre, disminución del apetito, aumento de la acidez de la sangre, otros cambios en las sales de la sangre (ver análisis de sangre).
- Síntomas de ansiedad, confusión y desorientación, depresión, cambios en el estado de ánimo, pesadillas, alucinaciones, trastornos mentales.
- Ataques, trastornos del nivel de conciencia, hormigueos y entumecimiento (a veces doloroso) en las manos y los pies, mareos, disminución de la capacidad para escribir, trastornos del sistema nervioso.
- Aumento de la sensibilidad a la luz, trastornos oculares.
- Zumbidos en los oídos.
- Reducción del flujo sanguíneo en los vasos cardíacos, latido cardíaco más rápido.
- Sangrado, bloqueo parcial o completo de los vasos sanguíneos, reducción de la presión arterial.
- Falta de aliento, cambios en el tejido pulmonar, acumulación de líquido alrededor de los pulmones, inflamación de la garganta, tos, síntomas de tipo gripal.
- Inflamaciones o úlceras que producen dolor abdominal o diarrea, sangrado en el estómago, inflamaciones o úlceras en la boca, acumulación de líquido en la tripa, vómitos, dolores abdominales, indigestión, estreñimiento, flatulencia, hinchazón abdominal, heces sueltas, problemas de estómago.
- Trastornos del conducto biliar, coloración amarillenta de la piel debido a problemas hepáticos, daño tisular hepático e inflamación hepática.
- Picor, erupción, pérdida de pelo, acné, aumento de la sudoración.
- Dolor en las articulaciones, extremidades, espalda y pies, espasmos musculares.
- Función insuficiente de los riñones, reducción de la producción de orina, trastornos o dolor al orinar.
- Debilidad general, fiebre, acumulación de líquido en el organismo, dolor y molestias, aumento de la enzima fosfatasa alcalina en la sangre, aumento de peso, sensación de temperatura alterada.
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Cambios en la coagulación sanguínea, reducción en el número de todos los tipos de células sanguíneas (ver análisis de sangre).
- Deshidratación.
- Reducción de las proteínas o el azúcar en la sangre, aumento del fosfato en la sangre.
- Coma, sangrado cerebral, ictus, parálisis, trastornos cerebrales, anomalías del habla y el lenguaje, problemas de memoria.
- Opacidad del cristalino.
- Deterioro de la audición.
- Latido irregular, parada del latido cardíaco, disminución del rendimiento del corazón, trastornos del músculo cardíaco, aumento de tamaño del músculo cardíaco, latido más fuerte, ECG anormal, frecuencia cardiaca y pulso anormales.
- Coágulo sanguíneo de una vena de un miembro, shock.
- Dificultades para respirar, trastornos de las vías respiratorias, asma.
- Obstrucción del intestino, aumento del nivel sanguíneo de la enzima amilasa, reflujo del contenido del estómago en su garganta, retraso en el vaciamiento del estómago.
- Inflamación de la piel, sensación de quemadura a la luz del sol.
- Trastornos articulares.
- Incapacidad para orinar, menstruación dolorosa y sangrado menstrual anormal.
- Fallo multiorgánico, enfermedad tipo gripal, aumento de la sensibilidad al calor y al frío, sensación de presión en el tórax, inquietud o sensación anormal, aumento de la enzima lactato deshidrogenasa en la sangre, pérdida de peso.
Efectos adversos raros (pueden afectar hasta 1 de cada 1.000 personas):
- Pequeños sangrados de la piel por coágulos sanguíneos.
- Aumento de la rigidez muscular.
- Sordera.
- Acumulación de líquido alrededor del corazón.
- Falta de aliento aguda.
- Formación de quistes en el páncreas.
- Problemas con el flujo sanguíneo en el hígado.
- Aumento de la vellosidad.
- Sed, caídas, sensación de opresión en el pecho, disminución de la movilidad, úlcera.
Efectos adversos muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- Debilidad muscular.
- Ecografía cardiaca anormal.
- Insuficiencia hepática.
- Dolor al orinar, con sangre en la orina.
- Aumento del tejido graso.
Niños y adolescentes
Los niños y adolescentes pueden experimentar los mismos efectos adversos que los adultos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Modigraf
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el sobre después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación.
Después de la preparación, la suspensión debe administrarse inmediatamente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Modigraf
- El principio activo es tacrólimus.
Cada sobre de Modigraf 0,2 mg granulado contiene 0,2 mg de tacrólimus (como monohidrato).
Cada sobre de Modigraf 1 mg granulado contiene 1 mg de tacrólimus (como monohidrato).
- Los demás componentes son: lactosa monohidrato, hipromelosa (E464) y croscarmelosa sódica (E468).
Aspecto del producto y contenido del envase
Modigraf granulado para suspensión oral son gránulos blancos suministrados en sobres.
Se comercializan cajas con 50 sobres.
Titular de la Autorización de Comercialización
Astellas Pharma Europe B.V.
Sylviusweg 62
2333 BE Leiden
Países Bajos
Responsable de la fabricación
Astellas Ireland Co., Ltd.
Killorglin
County Kerry, V93FC86
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Astellas Pharma B.V. Branch Tél/Tel: + 32 (0)2 5580710 | Lietuva Astellas Pharma d.o.o. Tel: +370 37 408 681 |
| Luxembourg/Luxemburg Astellas Pharma B.V. Branch Belgique/Belgien Tél/Tel: + 32 (0)2 5580710 |
Ceská republika Astellas Pharma s.r.o. Tel: +420 221 401 500 | Magyarország Astellas Pharma Kft. Tel.: + 36 1 577 8200 |
Danmark Astellas Pharma a/s Tlf: + 45 43 430355 | Malta Astellas Pharmaceuticals AEBE Tel: +30 210 8189900 |
Deutschland Astellas Pharma GmbH Tel: + 49 (0)89 454401 | Nederland Astellas Pharma B.V. Tel: + 31 (0)71 5455745 |
Eesti Astellas Pharma d.o.o. Tel: +372 6 056 014 | Norge Astellas Pharma Tlf: + 47 66 76 46 00 |
Ελλ?δα Astellas Pharmaceuticals AEBE Τηλ: +30 210 8189900 | Österreich Astellas Pharma Ges.m.b.H. Tel: + 43 (0)1 8772668 |
España Astellas Pharma S.A. Tel: + 34 91 4952700 | Polska Astellas Pharma Sp.z.o.o. Tel.: + 48 225451 111 |
France Astellas Pharma S.A.S. Tél: + 33 (0)1 55917500 | Portugal Astellas Farma, Lda. Tel: + 351 21 4401320 |
Hrvatska Astellas d.o.o. Tel: + 385 1 670 01 02 | România S.C. Astellas Pharma SRL Tel: +40 (0)21 361 04 95 |
Ireland Astellas Pharma Co. Ltd. Tel: + 353 (0)1 4671555 | Slovenija Astellas Pharma d.o.o. Tel: +386 (0) 14011 400 |
Ísland Vistor hf Sími: + 354 535 7000 | Slovenská republika Astellas Pharma s.r.o., Tel: +421 2 4444 2157 |
Italia Astellas Pharma S.p.A. Tel: + 39 02 921381 | Suomi/Finland Astellas PharmaPuh/Tel: + 358 (0)9 85606000 |
Κ?προς Astellas Pharmaceuticals AEBE Ελλ?δα Τηλ: +30 210 8189900 | Sverige Astellas Pharma AB Tel: + 46 (0)40-650 15 00 |
Latvija Astellas Pharma d.o.o. Tel: +371 67 619365 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MODIGRAF 0,2 mg GRANULADO PARA SUSPENSION ORALForma farmacéutica: CAPSULA, 0,5 mgPrincipio activo: TacrolimusFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: CAPSULA, 1 mgPrincipio activo: TacrolimusFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: CAPSULA, 2 mgPrincipio activo: TacrolimusFabricante: Sandoz Farmaceutica S.A.Requiere receta
Médicos online para MODIGRAF 0,2 mg GRANULADO PARA SUSPENSION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MODIGRAF 0,2 mg GRANULADO PARA SUSPENSION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







