LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 MG POLVO Y DISOLVENTE PARA SUSPENSION DE LIBERACION PROLONGADA INYECTABLE

Cómo usar LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 MG POLVO Y DISOLVENTE PARA SUSPENSION DE LIBERACION PROLONGADA INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Leuprorelina GP-Pharm Depot Trimestral y para qué se utiliza
- Qué necesita saber antes de empezar a usar Leuprorelina GP-Pharm Depot Trimestral
- Cómo usar Leuprorelina GP-Pharm Depot Trimestral.
- Posibles efectos adversos
- Conservación de Leuprorelina GP-Pharm Depot Trimestral
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Leuprorelina GP-Pharm Depot Trimestral 22,5 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Acetato de leuprorelina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Leuprorelina GP-Pharm Depot Trimestral y para qué se utiliza
- Qué necesita saber antes de empezar a usar Leuprorelina GP-Pharm Depot Trimestral
- Cómo usar Leuprorelina GP-Pharm Depot Trimestral
- Posibles efectos adversos
- Conservación de Leuprorelina GP-Pharm Depot Trimestral
- Contenido del envase e información adicional
1. Qué es Leuprorelina GP-Pharm Depot Trimestral y para qué se utiliza
Leuprorelina GP-Pharm Depot Trimestral es un vial que contiene un polvo blanco, que se reconstituye en forma de suspensión para su inyección en un músculo. Este medicamento contiene el principio activo leuprorelina (también conocido como leuprolide), que pertenece a un grupo de medicamentos llamado agonistas de la hormona liberadora de gonadotropina (LHRH), que son medicamentos que reducen la testosterona (una hormona sexual).
Su médico le ha recetado este medicamento para el tratamiento paliativo del cáncer de próstata avanzado.
2. Qué necesita saber antes de empezar a usar Leuprorelina GP-Pharm Depot Trimestral
No use Leuprorelina GP-Pharm Depot Trimestral:
- Si es alérgico (hipersensible) a la LHRH, a los agonistas de la LHRH o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6). Una reacción alérgica puede manifestarse como erupción cutánea, picor, dificultad para respirar o hinchazón de la cara, labios, garganta o lengua.
- Si se ha sometido a una orquiectomía (resección de los testículos).
- Si es una mujer o un niño.
- Este medicamento no debe utilizarse solo (en monoterapia) para el tratamiento del cáncer de próstata cuando la médula espinal está comprimida o el cáncer se ha extendido hasta la médula.
Advertencias y precauciones
- Consulte a su médico o farmacéutico antes de empezar a tomar este medicamento.
- Es posible que su enfermedad empeore durante las primeras semanas del tratamiento, pero deberá mejorar con el tratamiento continuado. Los signos y síntomas incluyen: aumento temporal de la testosterona (hormona masculina), sofocos, dolor óseo, trastornos del sistema nervioso (incluyendo depresión) u obstrucciones urinarias.
- Si cree que está experimentando una reacción alérgica (falta de aliento, asma, rinitis, hinchazón de la cara, urticaria, erupción cutánea), deje de tomar este medicamento e informe a su médico.
- Informe a su médico si tiene riesgo de padecer, o padece ya, alguna de las siguientes enfermedades, ya que puede necesitar revisiones más frecuentes:
- Hematomas o sangrado sin explicación o si experimenta malestar general. Aunque es raro, estos pueden ser síntomas de cambios en el número de glóbulos rojos o blancos
- Enfermedad metabólica
- Problemas del corazón, o latido de corazón palpitante
- Diabetes.
- El médico debe ser informado de cualquier antecedente clínico personal de adenoma hipofisario (tumor no maligno de la hipófisis). Se han descrito casos de apoplejía hipofisaria (pérdida parcial de tejido de la hipófisis) tras la administración inicial de este tipo de medicamento a pacientes con adenoma hipofisario. Puede manifestarse apoplejía hipofisaria, en forma de dolor de cabeza repentino, meningismo, trastornos de la visión o visión alterada, incluida ceguera, y ocasionalmente una disminución del nivel de consciencia.
- Su médico deberá saber si usted sufre de un trastorno de la coagulación, trombocitopenia o si usted está en tratamiento con anticoagulantes. Es posible que su función hepática deba supervisarse, ya que se han descrito alteraciones del hígado e ictericia (coloración amarillenta de los ojos y la piel) con la administración de leuprorelina.
- Se ha descrito fractura de la columna, parálisis, presión arterial baja y presión arterial alta con el tratamiento con leuprorelina.
- Se han notificado casos de depresión en pacientes en tratamiento con este medicamento, que puede ser severa. Si usted está usando este medicamento y se siente deprimido, informe a su médico.
- Se ha descrito reducción de la densidad ósea (huesos frágiles o más finos) tras la administración de leuprorelina. El médico puede considerar la posibilidad de añadir un antiandrógeno al tratamiento con este medicamento. En este caso, el médico estará alerta para detectar la presencia de inflamación de las venas (tromboflebitis) y otros signos de trastornos de la coagulación y edema (hinchazón de manos, pies o tobillos) que tienen más riesgo de producirse cuando se añade tratamiento antiandrogénico a este medicamento.
- Informe a su médico si siente presión en la médula espinal y/o presenta trastornos urinarios y/o hematuria (sangre en la orina); en tal caso, el médico le comentará la necesidad de tratamientos adicionales para prevenir complicaciones neurológicas (por ejemplo, hormigueo en manos y pies, parálisis) u obstrucción de la uretra (conducto que conecta la vejiga con el exterior del cuerpo). Se le supervisará estrechamente durante las primeras semanas de tratamiento.
- Los pacientes pueden experimentar cambios metabólicos (por ejemplo, intolerancia a la glucosa o empeoramiento de la diabetes existente), cambios de peso y trastornos cardiovasculares.
- Los pacientes con enfermedad metabólica o cardiovascular, y especialmente los pacientes con antecedentes de insuficiencia cardiaca congestiva (enfermedad en la que el corazón ya no puede bombear suficiente sangre al resto del cuerpo), deberán ser controlados durante el tratamiento con leuprorelina.
- Consulte a su médico, farmacéutico o enfermero si tiene hígado graso.
- Durante el tratamiento deberá realizarse algunos análisis de sangre para comprobar si este medicamento es eficaz.
- Usted puede experimentar una pérdida de interés en las relaciones sexuales, sofocos y ocasionalmente puede producirse una reducción en el tamaño y la función de los testículos.
- Puede volver a ser fértil de nuevo cuando se interrumpa el tratamiento con este medicamento.
- Este medicamento puede interferir con ciertas pruebas analíticas, por lo que deberá asegurarse de que su médico conoce que está usando este medicamento.
- Pueden producirse convulsiones en pacientes predispuestos (pacientes con historial de convulsiones, epilepsia, trastornos cerebrovasculares, anomalías o tumores del sistema nervioso central), en pacientes que toman fármacos que pueden causar convulsiones y, en menor medida, en pacientes que no presentan estas características.
- Informe a su médico si padece alguna afección del corazón o los vasos sanguíneos o está siendo tratado para ello, incluyendo medicamentos para controlar el ritmo cardíaco (arritmias). El riesgo de problemas del ritmo cardíaco puede aumentar cuando se utiliza este medicamento.
- Póngase en contacto con su médico inmediatamente si padece cefaleas intensas o recurrentes, problemas visuales y acúfenos o zumbidos.
- Se han comunicado erupciones cutáneas graves, incluido síndrome de Stevens-Johnson/necrólisis epidérmica tóxica (SJS/NET) en asociación con leuprorelina. Suspenda el uso de leuprorelina y busque atención médica de inmediato si nota cualquiera de los síntomas relacionados con estas reacciones cutáneas graves descritas en la sección 4.
Uso de Leuprorelina GP-Pharm Depot Trimestral con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento. Es posible que siga siendo adecuado el tratamiento con este medicamento; el médico podrá decidir qué es adecuado para usted.
Este medicamento puede interferir con algunos medicamentos utilizados para tratar problemas del ritmo cardíaco (por ejemplo: quinidina, procainamida, amiodarona y sotalol) o puede aumentar el riesgo de problemas del ritmo cardíaco cuando se utiliza con otros medicamentos (por ejemplo: metadona (utilizado para el alivio del dolor y para la desintoxicación de otros medicamentos), moxifloxacino (un antibiótico), antipsicóticos usados para tratar enfermedades mentales graves).
Embarazo y lactancia
El uso de este medicamento no está indicado en mujeres.
Este medicamento está contraindicado durante el embarazo. Pueden producirse abortos espontáneos si este medicamento se administra durante el embarazo.
Conducción y uso de máquinas
No hay estudios específicos sobre los efectos de este medicamento sobre la capacidad para conducir y utilizar máquinas.
Pueden producirse alteraciones de la visión y mareos durante el tratamiento. Si se ve afectado, no conduzca ni maneje máquinas.
Leuprorelina GP-Pharm DepotTrimestralcontienemenos de 23 mg de sodio (1 mmol) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Leuprorelina GP-Pharm Depot Trimestral.
Posología
Este medicamento solo debe ser administrado por su médico o enfermero. Ellos serán quienes se encarguen de la preparación del producto.
Adultos y ancianos:
La dosis recomendada de este medicamento es de una inyección cada tres meses. El polvo se reconstituye para formar una suspensión que se administra en forma de inyección intramuscular (en un músculo) una vez cada tres meses.
El lugar de inyección deberá variar a intervalos regulares.
Este medicamento debe ser administrado únicamente por vía intramuscular. No se debe administrar por otra vía.
La pauta del tratamiento será decisión de su médico.
Uso en niños:Este medicamento no está indicado en niños.
Si usa más Leuprorelina GP-Pharm Depot Trimestral del que debe
Esto es improbable, ya que el médico o la enfermera sabrán cuál es la dosis adecuada. No obstante, si sospecha que ha recibido más medicamento del que debiera, informe a su médico inmediatamente para que puedan tomarse las medidas necesarias.
En caso de sobredosis o ingestión accidental consulte con el Servicio de Información Toxicológica, tel: 91 562 04 20, indicando el medicamento y la cantidad usada.
Si olvidó usar Leuprorelina GP-Pharm Depot Trimestral
Es importante que no se salte una dosis de este medicamento. Tan pronto como sepa que se ha saltado una inyección, póngase en contacto con su médico, quien le administrará la siguiente inyección.
Si interrumpe el tratamiento con Leuprorelina GP-Pharm Depot Trimestral
Puesto que el tratamiento médico implica la administración de este medicamento durante un largo periodo de tiempo, al interrumpirse el tratamiento puede experimentarse un agravamiento de los síntomas relacionados con la enfermedad. Por tanto, no debe interrumpir el tratamiento de forma prematura sin el permiso de su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico inmediatamente si nota cualquiera de los siguientes síntomas:
- sufre respiración sibilante, dificultad para respirar, hinchazón de los párpados, cara o labios, erupción cutánea o picor (especialmente si le afecta a todo el cuerpo) de forma repentina.
- Frecuencia no conocida (la frecuencia no puede estimarse a partir de los datos disponibles):
- Si nota que en el tronco tiene manchas circulares o en forma de diana, de color rojizo y no elevadas, a menudo con ampollas centrales, descamación de la piel, úlceras en la boca, la garganta, la nariz, los genitales y los ojos. Estas erupciones cutáneas graves pueden estar precedidas de fiebre y síntomas de tipo gripal (síndrome de Stevens-Johnson/necrólisis epidérmica tóxica).
- Enrojecimiento de la piel y erupción con picor (Erupción cutánea tóxica).
- Una reacción cutánea que provoca granos o manchas rojas en la piel, que pueden parecer una diana, con un centro rojo oscuro rodeado de anillos de color rojo más claro (eritema multiforme).
Se han descrito los siguientes efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
Sofocos y reacciones en el lugar de administración.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
Sudores fríos, hiperhidrosis (aumento de la sudoración), prurito (picor), fatiga, insomnio, disminución del deseo sexual, vértigo, rubor, sensación de mareo (nauseas), diarrea, disminución del apetito, disfunción eréctil, astenia (falta o pérdida de fuerza), dolor de huesos, dolor en las articulaciones y reacciones en el lugar de la inyección tales como dolor, irritación, eritema (enrojecimiento de la piel). Dolor del tracto urinario, flujo urinario disminuido, necesidad de orinar con frecuencia, cambios de humor y depresión en tratamientos prolongados con leuprorelina, alteración de las enzimas hepáticas, hiperlipidemia (niveles elevados de lípidos en sangre), aumento de azúcar en sangre.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
Colesterol elevado, trastornos del sueño, inquietud, alteración del gusto, hormigueo (alteración en la sensibilidad de la piel), dolor de cabeza, letargo (somnolencia), visión borrosa, pleuresía, zumbido en los oídos (tinnitus), dolor en el abdomen superior, estreñimiento, pápula, erupción, prurito generalizado (picor), sudores nocturnos, dolor de espalda, dolor muscular, dolor de cuello, dolor en las mamas, dolor pélvico, atrofia testicular, trastorno testicular, sensación de calor, cambios de humor y depresión en tratamientos a corto plazo con leuprorelina. Cambios enlos valores sanguíneos y en el electrocardiograma ECG (prolongación del intervalo QT). Reacciones en el lugar de inyección tales como urticaria, calor y hemorragia.
No conocida (la frecuencia no puede estimarse a partir de los datos disponibles)
Inflamación de los pulmones, enfermedad pulmonar
Hipertensión intracraneal idiopática (aumento de la presión intracraneal alrededor del cerebro caracterizado por cefaleas, diplopía y otros síntomas visuales, acúfenos o zumbidos en uno o los dos oídos).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Leuprorelina GP-Pharm Depot Trimestral
Su médico o farmacéutico sabrán cómo conservar este medicamento.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25ºC. No congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, vial y jeringa después de “CAD”. La jeringa tiene la misma caducidad que el vial.
La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Leuprorelina GP-Pharm Depot Trimestral
El principio activo es acetato de leuprorelina. Cada vial contiene 22,5 mg de acetato de leuprorelina.
La concentración del producto reconstituido es de 11,25 mg/ml. Los demás componentes son: polisorbato 80, manitol (E-421), carmelosa sódica (E-466), trietil citrato y poli(ácido láctico) (PLA).
El disolvente contiene (jeringa precargada): manitol, agua para preparaciones inyectables, hidróxido de sodio (para ajuste de pH) y ácido clorhídrico (para ajuste de pH).
Aspecto del producto y contenido del envase
Cada envase contiene un vial con 22,5 mg de acetato de leuprorelina, una jeringa precargada con 2 ml de disolvente, un sistema adaptador y una aguja estéril del calibre 20.
Titular de la autorización de comercialización y responsable de la fabricación
GP-PHARM, S.A.
Pol. Ind. Els Vinyets – Els Fogars Sector 2
Carretera comarcal 244, km22
08777 Sant Quintí de Mediona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
España: Leuprorelina GP-Pharm Depot Trimestral 22.5 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Alemania: Lutrate Depot 22.5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-Injektionssuspension
Portugal: Lutrate Depot 22.5 mg / 2 ml pó e veículo para suspensão injectável de libertação prolongada
Grecia: Lutrate Depot 22.5mg Κ?νις και διαλ?της για παρασκευ? ενεσ?μου εναιωρ?ματος παρατεταμ?νης αποδ?σμευσης
Italia: Politrate
Hungria: Politrate Depot 22.5 mg
Austria: Lutrate 3-Monats-Depot 22.5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-injektionssuspension
República Checa: Lutrate Depot 22.5mg
Polonia: Lutrate Depot
Bulgaria: ?????? ???? 22,5 mg ???? ? ??????????? ?? ??????????? ????????? ? ???????? ?????????????
Fecha de la última revisión de este prospecto:Abril 2025.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
Esta información está destinada únicamente a profesionales del sector sanitario.
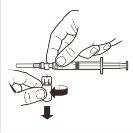
¿Cómo preparar la inyección?
IMPORTANTE: Lea detenidamente antes de administrar el producto (las Instrucciones de uso también se incluyen en la bandeja que contiene los componentes del kit).
Deberá seguirse una técnica aséptica durante el procedimiento de reconstitución.
Utilice únicamente el solvente incluido en el kit comercial.
Una vez mezclado, el producto debe administrarse inmediatamente por inyección intramuscular única.
Este medicamento es de un solo uso. Cualquier resto de suspensión debe ser desechado.
Compruebe el contenido del kit y asegúrese de que incluye todo lo mencionado en el prospecto.
El envase contiene:
1 (un) vial de Leuprorelina GP-Pharm Depot 22,5 mg (acetato de leuprorelina) polvo para suspensión inyectable
1 (una) jeringa precargada que contiene el disolvente para la suspensión (solución inyectable de manitol 0,8%)
1 (un) dispositivo para la reconstitución estéril de un solo uso, incluyendo 1 (una) aguja estéril.
1 |
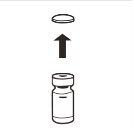
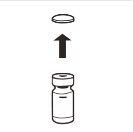
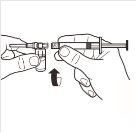
| Retire por completo la tapa de cierre a presión de la parte superior del vial, de modo que el tapón de goma quede al descubierto. Confirme que no quedan partes de la tapa de cierre a presión en el vial. |
2 |
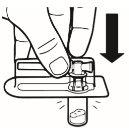
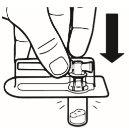
| Coloque el vial en posición vertical sobre una mesa. Quite la cubierta del blíster que contiene el adaptador del vial (MIXJECT). No retire el adaptador del vial del blíster.Coloque firmemente el blíster que contiene el adaptador del vial en la parte superior del vial, perforando el tapónen posición totalmente vertical. Presione suavemente hacia abajohasta que note que encaja en su posición. |
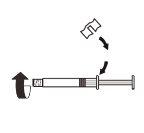
3 |
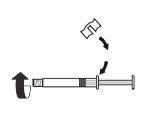
| Fije la pieza blanca a la jeringa hasta que note que encaja. Desenrosqueel tapón rígido de la jeringa en sentido antihorario. Después, retire el blíster del sistema adaptador MIXJECT. |
4 |
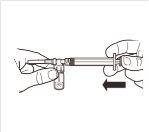
| Conecte la jeringa al sistema adaptador enroscándola en sentido horario en la apertura lateral del sistema adaptador. Para asegurar una conexión hermética, enrosque suavemente la jeringa hasta que se detenga. |
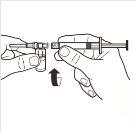
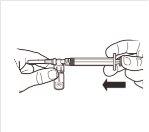
5 |
| Mientras mantiene la jeringa y el vial firmemente unidos en posición vertical, empuje lentamente el émbolo de la jeringa para transferir todo el disolvente al vial. |
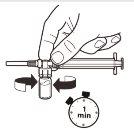
6 |
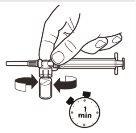
| Con la jeringa aún unida al vial, agite suavemente el vial durante un minuto aproximadamentehasta obtener una suspensión lechosa uniforme.Para evitar la separación de la suspensión, realice los siguientes pasos sin detenerse. |
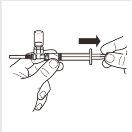
7 |
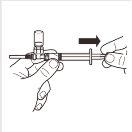
| Girar el sistema adaptador MIXJECT para que el vial se encuentre en la parte superior. Sujete firmemente el sistema adaptador MIXJECT por la jeringa y tire lentamente del émbolo para transferir el contenido del vial a la jeringa. Parte del producto puede acumularse o quedar depositado en la pared del vial. Esto es normal. |
8 |
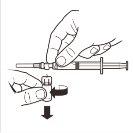
| Desconecte la jeringa del sistema adaptador MIXJECT. Para ello sujete firmemente la jeringa y gire el vial en sentido horario (sujetando por el tapón de plástico del sistema adaptador). |
9 |
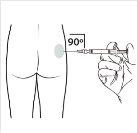
| Mantenga la jeringa EN POSICIÓN VERTICAL. Con la mano contraria quite el protector de la aguja tirando hacia arriba. Presione un poco el émbolo para expulsar el aire de la jeringa. La jeringa conteniendoel producto está preparada para su administración inmediata. |
10 |
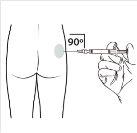
| Administre la inyección intramuscular insertando la aguja en un ángulo de 90 grados en glúteo. Asegúresede que la totalidad del producto sea inyectada.Las zonas de inyección deberían alternarse. |
Instrucciones de uso
Para incluir en la tapa de la bandeja que contiene los componentes del Kit del Medicamento
Leuprorelina GP-PharmDepot –Instrucciones de uso
Leer detenidamente antes de administrar el producto
Reconstituya inmediatamente antes de administrar por inyección intramuscular única
Utilice únicamente el solvente incluido en el kit comercial.
Producto destinado a una única inyección.
Cualquier resto de suspensión debe ser desechado.
1 |
| Retire completamente la tapa de cierre a presión de la parte superior del vial, de modo que el tapón de goma quede al descubierto. Confirme que no quedan partes de la tapa de cierre a presión en el vial. |
2 |
| Coloque el vial en posición vertical sobre una mesa. Quite la cubierta del blíster que contiene el adaptador del vial (MIXJECT). No retire el adaptador del vial del blíster.Coloque firmemente el blíster que contiene el adaptador del vial en la parte superior del vial, perforando el tapónen posición totalmente vertical. Presione suavemente hacia abajohasta que note que encaja en su posición. |
3 |
| Fije la pieza blanca a la jeringa hasta que note que encaja. Desenrosqueel tapón rígido de la jeringa en sentido antihorario. Después, retire el blíster del sistema adaptador MIXJECT. |
4 |
| Conecte la jeringa al sistema adaptador enroscándola en sentido horario en la apertura lateral del sistema adaptador. Para asegurar una conexión hermética, enrosque suavemente la jeringa hasta que se detenga. |
5 |
| Mientras mantiene la jeringa y el vial firmemente unidos en posición vertical, empuje lentamente el émbolo de la jeringa para transferir todo el disolvente al vial. |
6 |
| Con la jeringa aún unida al vial, agite suavemente el vial durante un minuto aproximadamentehasta obtener una suspensión lechosa uniforme.Para evitar la separación de la suspensión, realice los siguientes pasos sin detenerse. |
7 |
| Girar el sistema adaptador MIXJECT para que el vial se encuentre en la parte superior. Sujete firmemente el sistema adaptador MIXJECT por la jeringa y tire lentamente del émbolo para transferir el contenido del vial a la jeringa. Parte del producto puede acumularse o quedar depositado en la pared del vial. Esto es normal. |
8 |
| Desconecte la jeringa del sistema adaptador MIXJECT. Para ello sujete firmemente la jeringa y gire el vial en sentido horario (sujetando por el tapón de plástico del sistema adaptador). |
9 |
| Mantenga la jeringa EN POSICIÓN VERTICAL. Con la mano contraria quite el protector de la aguja tirando hacia arriba. Presione un poco el émbolo para expulsar el aire de la jeringa. La jeringa conteniendo el productoestá preparada para su administración inmediata. |
10 |
| Administre la inyección intramuscular insertando la aguja en un ángulo de 90 grados en glúteo. Asegúresede que la totalidad del producto sea inyectada.Las zonas de inyección deberían alternarse. |
- País de registro
- Precio medio en farmacia301.02 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 MG POLVO Y DISOLVENTE PARA SUSPENSION DE LIBERACION PROLONGADA INYECTABLEForma farmacéutica: INYECTABLE, 42 mgPrincipio activo: leuprorelinFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE, 45 mgPrincipio activo: leuprorelinRequiere recetaForma farmacéutica: INYECTABLE, 22,5 mgPrincipio activo: leuprorelinRequiere receta
Médicos online para LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 MG POLVO Y DISOLVENTE PARA SUSPENSION DE LIBERACION PROLONGADA INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 MG POLVO Y DISOLVENTE PARA SUSPENSION DE LIBERACION PROLONGADA INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes