
LOCETAR 50 MG/ML BARNIZ DE UÑAS MEDICAMENTOSO

Cómo usar LOCETAR 50 MG/ML BARNIZ DE UÑAS MEDICAMENTOSO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Locetar barniz de uñas medicamentoso y para qué se utiliza
- Qué necesita saber antes de usar Locetar barniz de uñas medicamentoso
- Cómo usar Locetar barniz de uñas medicamentoso
- Posibles efectos adversos
- Conservación de Locetar barniz de uñas medicamentosos
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Locetar 50 mg/ml barniz de uñas medicamentoso
Amorolfina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas, ya que puede perjudicarles
- Si experimenta efectos adversos, consulte a su médico, o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 3 meses .
Contenido del prospecto
- Qué es Locetar barniz de uñas medicamentoso y para qué se utiliza.
- Qué necesita saber antes de empezar a usar Locetar barniz de uñas medicamentoso.
- Cómo usar Locetar barniz de uñas medicamentoso.
- Posibles efectos adversos.
- Conservación de Locetar barniz de uñas medicamentoso.
- Contenido del envase e información adicional.
1. Qué es Locetar barniz de uñas medicamentoso y para qué se utiliza
Locetar contiene amorolfina como principio activo, que pertenece a un grupo de medicamentos conocidos como antinfúngicos (empleados para tratar infecciones producidas por hongos y levaduras).
Este medicamento está indicado en el tratamiento de infecciones producidas por hongos en las uñas, de gravedad leve a moderada, en adultos.
Debe consultar a un médico si empeora o si no mejora después de 3 meses
2. Qué necesita saber antes de usar Locetar barniz de uñas medicamentoso
Se considera una infección por hongos de carácter leve a moderada, sin afectación de la matriz de la uña (lúnula), aquella en la que hay una decoloración de la uña (blanca, amarilla o marrón) y engrosamiento, aunque la apariencia puede variar considerablemente.
Si la infección se limita a la parte superior de la uña como en la imagen número 1 ó 2, donde no hay afectación de la matriz de la uña, sólo decoloración (blanca, amarilla o marrón), consulte a su farmacéutico.
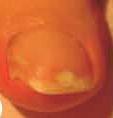
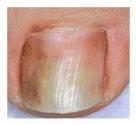


Si la infección tiene apariencia como en la foto 3 ó 4, donde se puede apreciar afectación de la matriz y rotura de la uña, consulte con su médico.

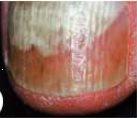


No use Locetar barniz de uñas medicamentoso
- si es alérgico a la amorolfina o a cualquiera de los demás componente de este medicamento (incluidos en la sección 6).
Niños y adolescentes
No se recomienda el uso de este medicamento en niños y adolecentes (de 12 a 18 años) por no haber suficiente experiencia clínica en este grupo de edad.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento.
- Si es un paciente con antecedentes de diabetes, trastornos inmunológicos(enfermedades que reducen las defensas del cuerpo), trastornos vasculares periféricos, heridas, dolor en las uñas o uñas seriamente dañadas, alteraciones de la piel como la psoriasis o cualquier alteración crónica de la piel, edema, trastornos de la respiración (síndrome de la uña amarilla).
- Si es diabético, tenga cuidado al recortar las uñas
- Si desarrolla sensibilidad al producto, deje el tratamiento y consulte a su médico.
- Durante el tratamiento no debe utilizar uñas artificiales.
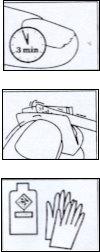
- Después de aplicar Locetar, se debe respetar un intervalo de al menos 10 minutos antes de la aplicación de cualquier esmalte cosmético.
- Antes de repetir la aplicación de Locetar, el esmalte cosmético debe eliminarse cuidadosamente.
- En pacientes en tratamiento, que trabajen habitualmente con solventes orgánicos, se recomienda protección de las manos con guantes impermeables.
- Evite el contacto con ojos y mucosas. Si accidentalmente se produjese contacto con los ojos, lavar con agua abundante y consultar con un oftalmólogo si fuese necesario.
Este medicamento puede causar reacciones alérgicas, algunas pueden ser serias. Si esto ocurre, interrumpa el tratamiento, elimine el medicamento inmediatamente con quitaesmaltes y solicite consejo médico. El medicamento no debe volver a aplicarse.
Debe conseguir ayuda médica urgente si tiene cualquiera de los siguientes síntomas:
- Dificultad para respirar.
- Su cara, labios, lengua o garganta están hinchados.
- Su piel ha desarrollado una erupción intensa.
Uso deLocetarbarniz de uñas medicamentoso con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Embarazo
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. El tratamiento con Locetar sólo se realizará, si se necesita de urgencia, después de que el responsable médico haya evaluado cuidadosamente los beneficios frente a los posibles riesgos.
Lactancia
Se desconoce si es segura la aplicación de este medicamento en mujeres en periodo de lactancia. El tratamiento con Locetar sólo se realizará, si se necesita de urgencia, después de que el responsable médico haya evaluado cuidadosamente los beneficios frente a los posibles riesgos.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir o utilizar máquinas es nula o insignificante.
Locetar contiene alcohol (etanol)
Este medicamento contiene 481,3 mg de alcohol (etanol) en cada ml de solución. Puede causar sensación de ardor en la piel lesionada.
Locetar contiene etanol, el cual es inflamable y no debe utilizarse cerca de una llama, cigarrillos encendidos o algunos dispositivos (por ejemplo, secadores de pelo).
3. Cómo usar Locetar barniz de uñas medicamentoso
Siga exactamente las instrucciones de administración de este medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo con su médico o farmacéutico.
Forma de administración: uso cutáneo (sólo para uso sobre las uñas, no debe aplicarse en la piel de alrededor de las uñas).
Locetar sólo debe ser utilizado por adultos.
Aplicar la solución, 1 o 2 veces por semana, sólo sobre las uñas afectadas de las manos o de los pies. Intente asociar el uso del barniz medicamentoso a un hábito de higiene, en 1 día de la semana que usted elija y mantenga esta rutina mientras dure el tratamiento.
La duración requerida del tratamiento dependerá básicamente de la gravedad y localización de la infección. Por lo general, será de 6 meses en las uñas de las manos y de 9 a 12 meses en las de los pies.
Si los síntomas empeoran o aparecen nuevos síntomas se debe reevaluar la situación clínica . En el caso de no observar mejoría en 3 meses de tratamiento, se deberá consultar con un médico.
 Modo de aplicación:
Modo de aplicación:

Atención:
Las uñas sanas no deben limarse nunca con la lima utilizada para las uñas enfermas. Las infecciones por hongos son contagiosas. Para prevenir la infección, evite que otra persona use dicha lima.

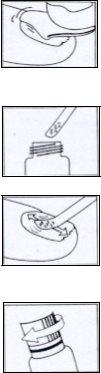

Se deben aplicar medidas generales de higiene con el fin de evitar la aparición de otras infecciones o recaídas.
Se recomienda el recorte regular de las uñas para eliminar las partes de la uña infectada.
En el caso de co-existencia de tinea pedis, ésta debe ser tratada con la crema antifúngica adecuada.
En caso de duda, se debe consultar con un médico o farmacéutico.
Si usa más Locetar barniz de uñas medicamentoso del que debe
Este medicamento no debe ser ingerido. ES SOLAMENTE PARA USO EXTERNO.
En caso de sobredosis o ingestión accidental, consulte inmediatamente con su médico o farmacéutico, o acuda a un centro médico, o llame al Servicio de Información Toxicológica, teléfono: 91 5620420, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Locetar barniz de uñas medicamentoso
No aplique una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Locetar barniz de uñas medicamentoso
Si interrumpe el tratamiento con Locetar antes de que su(s) uña(s) estén limpias o casi limpias, los hongos pueden no haber desaparecido. En este caso, la situación de sus uñas puede empeorar de nuevo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las reacciones adversas son raras. Pueden producirse alteraciones de las uñas (p.ej. decoloración de la uña, rotura de las uñas y uñas quebradizas). Estas reacciones pueden estar también relacionadas con la propia onicomicosis.
Raros (pueden afectar hasta 1 de cada 1.000 personas)
- Alteración de la uña,
- Decoloración de la uña,
- Onicoclasis (rotura de las uñas),
- Onicorrexis (uñas quebradizas).
Muy raros (pueden afectar hasta 1 de cada 10.000 personas)
- Sensación de quemazón en la piel
Frecuencia no conocida (no pueden estimarse a partir de los datos disponibles):
- Reacción alérgica sistémica (una reacción alérgica seria que puede asociarse con hinchazón de la cara, labios, lengua o garganta, dificultad para respirar y/o erupción cutánea intensa).
- Eritema, picor, dermatitis de contacto, urticaria y ampollas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Locetar barniz de uñas medicamentosos
Mantener este medicamento fuera de la vista y del alcance de los niños.
No requiere condiciones especiales de conservación.
Conservar en el embalaje original
No utilice este medicamento después de la fecha de caducidad indicada en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición Locetarbarniz de uñas medicamentoso
- El principio activo es amorolfina. Cada mililitro de solución contiene 50 miligramos de amorolfina.
- Los demás excipientes son ácido metacrílico copolímero, triacetina, acetato de butilo, acetato de etilo y etanol al 96 %.
Aspecto del producto y contenido del envase
Frasco de vidrio ambar con tapón de polipropileno (HDPE), que contiene 5 ml de solución.
El envase incluye: 30 toallitas limpiadoras, dentro de sobres individuales, así como 10 espátulas desechables y 30 limas.
Frasco de vidrio ambar con tapón de polipropileno (HDPE), que contiene 5 ml de solución y una espátula integrada en el tapón (LDPE),.
El envase incluye 30 toallitas limpiadoras, dentro de sobres individuales y 30 limas
Puede que solamente esté comercializado alguno de los envases.
Titular de la autorización de comercialización
Laboratorios Galderma, S.A.
Serrano Galvache, 56
28033 Madrid
España
Responsable de la fabricación
Laboratoires Galderma
Alby sur Chéran (Francia)
Fecha de última revisión de este prospecto:Octubre 2017
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/
.
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LOCETAR 50 MG/ML BARNIZ DE UÑAS MEDICAMENTOSOForma farmacéutica: BARNIZ DE UÑAS, 50 mg/mlPrincipio activo: amorolfineFabricante: Bluefish Pharmaceuticals Ab (Publ)Requiere recetaForma farmacéutica: BARNIZ DE UÑAS, 5% p/p Amorolfina (como clorhidrato)Principio activo: amorolfineFabricante: Isdin S.A.Requiere recetaForma farmacéutica: BARNIZ DE UÑAS, 5% p/p Amorolfina (como clorhidrato)Principio activo: amorolfineFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para LOCETAR 50 MG/ML BARNIZ DE UÑAS MEDICAMENTOSO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LOCETAR 50 MG/ML BARNIZ DE UÑAS MEDICAMENTOSO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








