KYLEENA 19,5 MG SISTEMA DE LIBERACION INTRAUTERINO

Cómo usar KYLEENA 19,5 MG SISTEMA DE LIBERACION INTRAUTERINO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Kyleena y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kyleena
- Su médico/profesional sanitario debe estar seguro de que este anticonceptivo es adecuado para usted .
- Como anticonceptivo, kyleena previene los embarazos. sin embargo, ningún anticonceptivo evita todos los embarazos .
- El SLI no le protege frente a la infección por el vih ni ninguna otra enfermedad de transmisión sexual
- ElSLIno es un anticonceptivo de emergencia como, por ejemplo, la píldora del día después .
- Cómo usar Kyleena
- Posibles efectos adversos
- Puede ocurrir que Kyleena se introduzca en la pared del útero o la atraviese. Esto se denomina perforación. Una perforación suele producirse en el momento de la inserción . Una perforación no siempre duele, por lo que es posible que solo la note más tarde. Si ya no está colocado en su sitio debido a la perforación, ya no es eficaz frente al embarazo. En ese caso, un médico/profesional sanitario debe retirárselo lo antes posible. A veces, es necesaria una intervención quirúrgica.
- Una perforación afecta hasta 1 de cada 1000personas. El riesgo de perforación es mayor (hasta 1 de cada 100personas) si:
- Conservación de Kyleena
- Contenido del envase e información adicional
Introducción
Prospecto: información para la usuaria
Kyleena 19,5mg sistema de liberación intrauterino
levonorgestrel
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico/profesional sanitario.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas.
- Si experimenta efectos adversos, consulte a su médico/profesional sanitario, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Kyleena y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kyleena
- Cómo usar Kyleena
- Posibles efectos adversos
- Conservación de Kyleena
- Contenido del envase e información adicional
1. Qué es Kyleena y para qué se utiliza
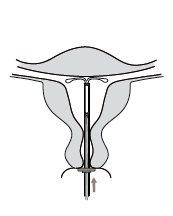
Kyleena es un sistema de liberación intrauterino (SLI) en forma de T, también conocido como dispositivo intrauterino hormonal. Es un método anticonceptivo que previene el embarazo con una duración de hasta 5 años. El SLI contiene una hormona llamada levonorgestrel.
Figura 1: Sistema de liberación intrauterino


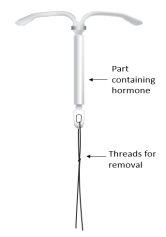
Cómo actúa
Un médico/profesional sanitario se lo insertará dentro del útero. Una vez insertado, libera continuamente una pequeña cantidad de la hormona.
Kyleena impide que el esperma y el óvulo entren en contacto y previene así el embarazo. Actúa de la siguiente forma:
- espesa el moco del cuello del útero (cérvix), lo que impide el paso de los espermatozoides.
- hace que el revestimiento del útero (endometrio) se mantenga fino.
Figura 2: Kyleena en el útero


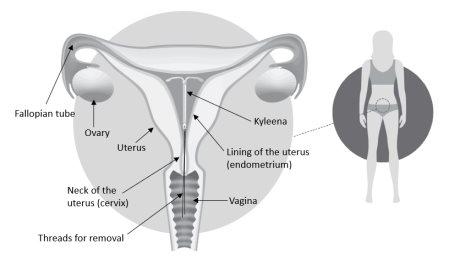
2. Qué necesita saber antes de empezar a usar Kyleena
Información importanteatener en cuenta Su médico/profesional sanitario debe estar seguro de que este anticonceptivo es adecuado para usted .Como anticonceptivo, kyleena previene los embarazos. sin embargo, ningún anticonceptivo evita todos los embarazos .El SLI no le protege frente a la infección por el vih ni ninguna otra enfermedad de transmisión sexualElSLIno es un anticonceptivo de emergencia como, por ejemplo, la píldora del día después . |
NO use Kyleena si:
- está embarazada (ver la sección “Embarazo, lactancia y fertilidad” que aparece más abajo);
- tiene una infección en el útero, las trompas de Falopio o los ovarios (enfermedad inflamatoria pélvica) o si ha tenido esta afección varias veces en el pasado;
- tiene una enfermedad que le hace más propensa a contraer infecciones pélvicas. Por ejemplo: enfermedades de transmisión sexual o enfermedades que reducen la capacidad del organismo para combatir infecciones como, por ejemplo, las fases avanzadas del VIH;
- tiene una infección en la vagina o el cuello del útero (cérvix);
- ha tenido un bebé o un aborto, espontáneo o no, durante los últimos 3 meses y posteriormente, ha tenido una infección en el útero;
- los resultados obtenidos en la última citología vaginal) fueron anómalos;
- tinee cáncer de útero o del cuello útero (cérvix) o su médico/profesional sanitario sospecha que puede tenerlo;
- tiene un tumor que necesita progestágenos para crecer - p. ej. cáncer de mama);
- tiene sangrados vaginales y se desconoce la causa;
- tiene un útero o un cuello uterino con forma anómala - p. ej., debido a crecimientos no cancerosos en el útero (mioma uterino);
- tiene una enfermedad hepática o un tumor hepático;
- es alérgica al levonorgestrel o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
No utilice Kyleena si alguno de los anteriores es aplicable en su caso. Si no está segura, hable con su médico/profesional sanitario.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Kyleena si usted:
- tiene diabetes. Por lo general no tendría que modificar su medicación antidiabética mientras utiliza el SLI, pero tal vez su médico/profesional sanitario deba comprobarlo;
- tiene epilepsia. Se puede producir un ataque (convulsión) durante la inserción o la retirada del SLI;
- ha tenido en el pasado un embarazo fuera del útero (embarazo ectópico);
- tiene migrañas que le provocan problemas de visión (p. ej., pérdida repentina de la visión en un ojo) u otro tipo de problemas (migrañas con aura) o si tiene dolores de cabeza intensos de origen desconocido;
- tiene ictericia (coloración amarillenta de la piel, las uñas y el blanco de los ojos);
- tiene la tensión arterial alta;
- alguna vez ha tenido un ictus o un ataque al corazón.
Si alguno de los anteriores es aplicable en su caso (o no está segura), hable con su médico/profesional sanitario antes de que le inserte Kyleena.
Mientras está utilizando Kyleena, hable con su médico/profesional sanitario inmediatamente si usted:
- tiene signos de embarazo o un resultado positivo en la prueba de embarazo (ver la sección “Embarazo, lactancia y fertilidad” más abajo);
- tiene signos de embarazo, pero también dolor, sangrado vaginal o se siente mareada. Esto puede indicar que está teniendo un embarazo fuera del útero (ver sección 4, apartado “Embarazo fuera del útero”);
- tiene dolor de estómago, fiebre o secreción vaginal inusual o dolor durante las relaciones sexuales. Esto puede indicar que tiene una infección y debe recibir tratamiento rápidamente (ver sección 4, apartado “Infección pélvica”);
- tiene dolor durante las relaciones sexuales, ya que podría tener un pequeño saco lleno de líquido (quiste) en el ovario (ver sección 4, apartado “Quiste ovárico”);
- tiene dolor intenso, sangrado muy abundante o ya no nota los hilos del SLI , ya que podría tener una perforación (ver sección 4, apartado “Perforación”).
Hable con su médico/profesional sanitario inmediatamente si presenta alguno de los síntomas anteriores.
Asimismo, hable con su médico/profesional sanitario acerca de Kyleena si usted:
- tiene una migraña o un dolor de cabeza muy intenso por primera vez;
- nota que la piel, las uñas y el blanco de los ojos se vuelven amarillos. Estos son signos de ictericia;
- nota un aumento de la tensión arterial;
- ha tenido un ictus o un ataque al corazón.
Su médico/profesional sanitario decidirá si sigue siendo seguro para usted seguir usando Kyleena.
Signos que podrían indicar que Kyleena no está en su sitio
Algunos signos que podrían indicar que el SLI no está en su sitio son:
- no poder palpar con el dedo los hilos en la vagina (ver sección 3, apartado “Cómo comprobar por sí misma si Kyleena está en su sitio”);
- notar la parte inferior de plástico (o su pareja la nota) (ver sección 3, apartado “Cómo comprobar por sí misma si Kyleena está en su sitio”);
- tener cambios repentinos en sus reglas (por ejemplo, ha dejado de tener la regla con el uso del SLI y después vuelve a tenerla de forma repentina).
Estos signos pueden significar que Kyleena se ha salido (ver sección 4, apartado “Si Kyleena se sale”) o que tiene una perforación (ver sección 4, apartado “Perforación”).
Si reconoce alguno de estos signos que indican que Kyleena no está en su sitio, hable con su médico/profesional sanitario inmediatamente. No debe mantener relaciones sexuales a menos que utilice un preservativo o un diafragma hasta que su médico/profesional sanitario compruebe que el SLI sigue en su sitio.
Es posible que su pareja note los hilos durante las relaciones sexuales. Esto no significa que el SLI esté fuera de su sitio. Sin embargo, si a su pareja le incomoda notar los hilos, hay cosas que su médico/profesional sanitario puede hacer para ayudarle.
Productos de higiene menstrual
Si tiene la regla, se recomienda el uso de compresas. Si usa tampones o una copa menstrual, debe cambiarlos con cuidado, de lo contrario prodría tirar accidentalmente de los hilos. Si cree que puede haber movido el SLI de su posición (consulte la lista anterior para conocer los posibles signos), no debe mantener relaciones sexuales a menos que use un preservativos o un diafragma hasta que acuda a su médico/profesional sanitario.
Problemas de salud mental
Algunas mujeres que utilizan anticonceptivos hormonales, incluido Kyleena, sufren depresión o estado de ánimo depresivo. Ver sección 4, apartado “Problemas de salud mental” para obtener más información.
Niños y adolescentes
Las chicas que todavía no tienen la regla no deben utilizar Kyleena.
Otros medicamentos y Kyleena
Informe a su médico/profesional sanitario si está tomando, ha tomado recientemente o pudiera tener que empezar a tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Embarazo
No se debe insertar Kyleena si está embarazada.
Si deja de tener la regla mientras utiliza Kyleena
Algunas mujeres no tienen la regla mientras lo utilizan. Si usted ya no tiene la regla, esto se debe probablement al SLI. Puede obtener más información al respecto en la sección 4, apartado “Sangrado irregular o poco frecuente”.
¿Hace 6 semanas que no tiene la regla? Entonces puede hacerse una prueba de embarazo. Si la prueba indica que no está embarazada, no hace falta repetirla.
Si nota síntomas de embarazo
Si presenta signos de embarazo como, por ejemplo, ausencia de reglas, ganas de vomitar o pechos sensibles o doloridos, debe:
- hacerse una prueba de embarazo;
- ponerse en contacto con su médico/profesional sanitario para que la examine.
Si se queda embarazada
Si se queda embarazada con Kyleena, acuda a su médico/profesional sanitario inmediatamente para que se lo retires.
Existe el riesgo de que sufra un aborto espontáneo durante su retirada . Sin embargo, si sigue utilizándolo a lo largo del embarazo, tiene más riesgo de:
- sufrir un aborto espontáneo;
- tener un bebé prematuro.
Hable con su médico/profesional sanitario sobre los beneficios y los riesgos de continuar con el embarazo mientras está utilizando el SLI. Su médico/profesional sanitario le vigilará estrechamente. Debe ponerse en contacto con su médico/profesional sanitario de inmediato si experimenta:
- retortijones;
- dolor de estómago;
- fiebre.
Kyleena contiene una hormona, llamada levonorgestrel. Pregunte a su médico/profesional sanitario sobre los efectos que la hormona podría tener en el feto. Se han notificado casos aislados de efectos en los genitales de bebés de sexo femenino expuestos a dispositivos intrauterinos de levonorgestrel mientras están en el útero.
Embarazo fuera del útero
El riesgo de que se quede embarazada mientras utiliza Kyleena es muy bajo. No obstante, si se queda embarazada mientras lo utiliza, tiene más riesgo de que el óvulo fecundado no esté en el útiero, sino en la trompa de Falopio o en la cavidad abdominal (embarazo ectópico). Un embarazo ectópico es un cuadro grave que precisa asistencia médica inmediata Tras sufrir un embarazo ectópico, puede resultar más difícil volver a quedarse embarazada. Ver sección 4, apartado “Embarazo fuera del útero”.
Lactancia
Puede utilizar Kyleena durante la lactancia. Una pequeña cantidad de la hormona puede pasar a la leche materna. No obstante, es poco probable que afecte a la cantidad o calidad de la leche materna o al crecimiento y desarrollo del lactante.
Fertilidad
Si se quiere quedar embarazada, debe ponerse en contacto con su médico/profesional sanitario para que le retire Kyleena.
Una vez retirado, no se ve afectada la fertilidad.
Conducción y uso de máquinas
Kyleena no tiene ninguna influencia conocida sobre la capacidad para conducir y utilizar máquinas.
3. Cómo usar Kyleena
Empezando a utilizar Kyleena
- Antes de insertarlo, debe asegurarse de no estar embarazada.
- Debe insertarse en el plazo de 7 días desde el inicio de la regla. Cuando se inserta durante esos días, actúa desde el momento de la inserción y evitará que se quede embarazada.
- Si no se lo pueden insertar en el plazo de 7 días desde el inicio de la regla o si su regla no es regular, entonces se puede insertar cualquier otro día. En este caso, no debe haber mantenido relaciones sexuales sin métodos anticonceptivos desde su última regla, así como tener una prueba de embarazo negativa antes de la inserción. Además, es posible que no prevenga de forma fiable el embarazo desde el momento de la inserción. Por tanto, debe utilizar un método anticonceptivo de barrera (como el preservativo) o abstenerse de mantener relaciones sexuales vaginales en los siguientes 7 días después de la inserción.
- Kyleena no es un anticonceptivo de emergencia como, por ejemplo, la píldora del día después.
Empezando a utilizar Kyleena después de dar a luz
- Puede insertarse tras dar a luz después de que el útero haya recuperado el tamaño normal, y no antes de 6 semanas después del parto (ver sección 4, apartado “Perforación”).
- Ver también “Empezando a utilizar Kyleena” arriba para ver qué más necesita saber sobre la elección del momento de la inserción.
Empezando a utilizar Kyleena después de un aborto
Se puede insertar inmediatamente después de un aborto si el embarazo ha durado menos de 3 meses, siempre que no haya infecciones genitales. El sistema actúa desde el momento de la inserción.
Empezando a utilizar un nuevo Kyleena cuando se debe reemplazar el actual en uso
Se puede sustituir en cualquier momento de su ciclo menstrual por otro nuevo. Actúa desde el momento de la inserción.
Cambio desde otro método anticonceptivo (como anticonceptivos hormonales combinados, implantes)
- Kyleena se puede insertar inmediatamente si se puede asegurar que usted no está embarazada.
- Si han pasado más de 7 días desde el inicio del sangrado menstrual, debe abstenerse de mantener relaciones sexuales vaginales o debe utilizar protección anticonceptiva adicional los siguientes 7 días.
Qué ocurre cuándo se inserta Kyleena?
Exploración antes de la inserción
Algunas veces, su médico/profesional sanitario querrá hacerle algún tipo de exploración antes de la inserción, entre ellas:
- una citología vaginal
- una exploración de las mamas
- otras pruebas (p. ej. para enfermedades de transmisión sexual), si es necesario.
Inserción de Kyleena
En primer lugar, su médico/profesional sanitario comprobará el tamaño del útero y su posición exacta en el abdomen (examen pélvico).
El médico/profesional sanitario introduce un instrumento llamado espéculo en la vagina y limpia el cuello del útero (cérvix) con un líquido antiséptico. Algunas veces, el profesional sanitario aplica anestesia local en el cérvix y, a continuación, inserta el SLI en el útero utilizando un tubo de plástico flexible y estrecho (tubo de inserción).
En algunas ocasiones, la inserción puede suponer molestias. Algunas mujeres experimentan mareo o desmayos. También puede experimentar dolor y algo de sangrado vaginal. Esto no es algo inusual.
Después de la inserción, el médico/profesional sanitario le entregará una tarjeta: la tarjeta recordatorio para la paciente. En esta tarjeta, puede anotar la fecha del siguiente chequeo. Lleve esta tarjeta en cada visita.
Revisión después de la inserción
Un profesional sanitario debe revisarle el SLI 4‑6 semanas después de su inserción. El médico/profesional sanitario determinará con qué frecuencia debe volver para una revisión posterior. Debe volver para que le revisen una vez al año como mínimo. Lleve la tarjetarecordatorio para la paciente a cada visita.
Cómo comprobar por sí misma si Kyleena está en su sitio
Puede comprobarlo introduciendo suavemente un dedo en la vagina. Debería notar los hilos en la parte superior de la vagina, cerca del cérvix. El cérvix es la entrada del útero. Nota: no tire de los hilos, ya que podría retirar el SLI accidentalmente.
Si no nota los hilos, debe pedir a su médico/profesional sanitario que compruebe si el SLI sigue todavía en su sitio. No debe mantener relaciones sexuales a menos que utilice un preservativo o un diafragma hasta que acuda a su médico/profesional sanitario.
Si usted o su pareja notan la parte inferior de plástico de Kyleena, éste no está en su sitio. Acuda a su médico/profesional sanitario de inmediato. No debe mantener relaciones sexuales hasta que haya acudido a su médico/profesional sanitario, a menos que utilice un preservativo o un diafragma.
Retirada de Kyleena
El SLI tiene una duración de hasta 5 años. Se lo deben retirar después de 5 años, aunque también se lo pueden retirar antes de que transcurran estos 5 años. Un sanitario profesional se lo retirará. Una vez retirado, se podrá volver a quedar embarazada.
Su retirada puede resultar algo molesta. Algunas mujeres experimentan mareo o desmayos durante la retirada o inmediatamente después. También puede experimentar algo de dolor y sangrado vaginal. Esto no es algo inusual.
Continuación de la anticoncepción después de la retirada
Si no desea quedarse embarazada tras la retirada de Kyleena, debe saber que:
- Es mejor retirarlo en los 7 días siguientes al inicio de la regla. Si se lo retiran en otro momento del ciclo menstrual, debe utilizar un preservativo o un diafragma durante las relaciones sexuales en los 7 días previos a la retirada .
- Si tiene reglas irregulares o ausencia de reglas, debe utilizar un preservativo o un diafragma durante las relaciones sexuales en los 7 días previos a la retirada. La aparición de reglas irregulares significa que el número de días entre ciclos mensuales no siempre es el mismo.
- También puede insertarse un nuevo SLI inmediatamente después de la retirada, en cuyo caso no hace falta protección adicional. Si no desea continuar con el mismo método anticonceptivo, pida consejo a su médico/profesional sanitario sobre otros métodos anticonceptivos fiables.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Kyleena puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Se pueden producir efectos adversos graves, por lo que debe ponerse en contacto con su médico/profesional sanitario inmediatamente si presenta cualquiera de los siguientes:
- Dolor de estómago, fiebre, secreción inusual o sangrado anormal de la vagina o dolor durante las relaciones sexuales. Puede tratarse de una infección en el útero, las trompas de Falopio o los ovarios (ver el apartado “Infección pélvica” más abajo).
- Dolor intenso similar al dolor menstrual, más dolor del esperado o sangrado vaginal muy abundante tras la inserción. O dolor o sangrados que duran más de unas semanas, cambios repentinos en el patrón menstrual, dolor durante las relaciones sexuales o incapacidad para notar los hilos del SLI. Estos pueden ser signos de una perforación (ver el apartado “Perforación” más abajo).
- Ausencia de la regla, pero después tiene un sangrado vaginal que no cesa, o dolor en la parte inferior del estómago que es intenso o no desaparece. Estos pueden ser signos de un embarazo fuera del útero (ver el apartado “Embarazo fuera del útero” más abajo).
- Tiene cambios de humor y síntomas depresivos (ver el apartado “Problemas de salud mental” más abajo).
- Reacción alérgica como, por ejemplo, erupción cutánea, urticaria o inflamación de la lengua, labios, cara o garganta. Este tipo de reacción es muy rara.
Si cree que alguno de los anteriores es aplicable en su caso, hable con su médico/profesional sanitario inmediatamente.
Otros efectos adversos
A continuación, se enumeran otros efectos adversos que puede presentar. Los efectos adversos que ocurren con más frecuencia se incluyen en la parte superior de esta lista, y los que ocurren con menos frecuencia se incluyen en la parte inferior.
Efectos adversos muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- dolor de cabeza
- dolor abdominal o pélvico
- granos (acné) o piel grasa
- cambios menstruales, por ejemplo:
- sangrar más o menos de lo habitual
- sangrar o sangrar un poco (manchado) sin tener la regla
- reglas irregulares o poco frecuentes
- ausencia de reglas
Puede obtener más información al respecto en la sección “Reglas irregulares o poco frecuentes” más abajo
- pequeño saco lleno de líquido en el ovario (quiste ovárico). Puede obtener más información al respecto en la sección “Quiste ovárico” más abajo
- inflamación de los labios y de la vagina (vulvovaginitis)
Efectos adversos frecuentes:pueden afectar hasta 1 de cada 10 personas
- disminución del deseo sexual
- migraña
- mareo
- ganas de vomitar (náuseas)
- pérdida de pelo
- dolor durante la regla
- dolor o sensibilidad en los pechos
- expulsión del SLI por sí solo (completa o parcialmente). Puede obtener más información al respecto en la sección “Si Kyleena se sale” más abajo
- secreción vaginal
- aumento de peso
Efectos adversos poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- aumento de vello corporal
Descripción de posibles efectos adversos seleccionados:
Embarazo fuera del útero
Los signos de un embarazo fuera del útero incluyen:
- ausencia de la regla, pero posteriormente se vuelven a producir sangrados vaginales persistentes
- dolor intenso o persistente en la parte baja del estómago
- signos normales del embarazo como, por ejemplo, náuseas o pechos sensibles o doloridos, pero también sangrado vaginal y sensación de mareo
- una prueba de embarazo positiva
Debe acudir a su médico/profesional sanitario inmediatamente si presenta alguno de estos síntomas.
El riesgo de que se quede embarazada mientras utiliza Kyleena es muy bajo. No obstante, si se queda embarazada mientras lo utiliza , tiene más riesgo de que el óvulo fecundado no esté en el útero, sino en las trompas de Falopio o en la cavidad abdominal (embarazo ectópico). Aproximadamente 2 de cada 1000 mujeres que lo utilizan durante un año acaban sufriendo un embarazo fuera del útero. Este tipo de embarazo es un cuadro grave que precisa atención médica inmediata. Es posible que necesite someterse a una intervención quirúrgica.
Algunas mujeres son más propensas a sufrir un embarazo ectópico, entre ellas mujeres que:
- han tenido un embarazo ectópico previo.
- se han sometido a una intervención quirúrgica en las trompas de Falopio.
- han tenido una infección pélvica.
Sangrado irregular o poco frecuente
Es probable que su regla sufra cabmios debido al SLU. Por ejemplo:
- Puede sangrar un poco cuando no esté con la regla. Este sangrado se denomina manchado.
- Puede tener la regla con menos regularidad. En ese caso, el número de días entre los ciclos menstruales no siempre es el mismo.
- Puede tener reglas más cortas o más largas.
- Puede perder más o menos sangre de lo habitual durante la regla.
- Puede no tener la regla.
A veces, estos cambios solo se producen en los primeros meses tras la inserción. Por ejemplo:
- Los sangrados cuando no se tiene la regla o los manchados son más frecuentes durante los primeros 3 a 6 meses.
- Algunas mujeres experimentan una regla más abundante de lo habitual al principio.
Es posible que gradualmente pierda menos sangre cada mes y tenga una regla más corta. Al final, algunas mujeres pueden dejar de tener la regla.
¿Ya no tiene la regla? Esto suele ser normal. La mayoría de las veces, esto no significa que esté embarazada o menopáusica. La razón es la siguiente: normalmente, el revestimiento del útero se engrosa cada mes para prepararse para un embarazo y luego se vuelve fino cuando tiene la regla. Kyleena reduce el engrosamiento del revestimiento uterino. Esto podría interrumpir la regla por completo. Sus propios niveles hormonales suelen permanecer normales.
Cuando se retira , normalmente vuelve a tener la regla con normalidad. Si no es así, póngase en contacto con su médico/profesional sanitario
Infección pélvica
Kyleena está libre de bacterias, virus y hongos (estéril). Esto también es aplicable al insertor. No obstante, aún puede contraer una infección en la pelvis durante la inserción o durante las 3 primeras semanas tras la inserción. Por ejemplo, en el revestimiento del útero, las trompas de Falopio o los ovarios. Esto puede afectar hasta 1 de cada 10 personas.
Puede presentar:
- dolor de estómago
- fiebre
- secreción vaginal inusual
- dolor durante las relaciones sexuales
Puede tener más riesgo de infección en la pelvis si:
- tiene una enfermedad de transmisión sexual
- usted o su pareja tienen varias parejas sexuales
- ya ha tenido una enfermedad en la pelvis anteriormente
Si tiene una infección pélvica, es importante acudir al médico/profesional sanitario inmediatamente. La infección pélvica puede provocar:
- problemas de fertilidad posteriores. Esto puede significar tener más dificultades para quedarse embarazada
- un embarazo fuera del útero (embarazo ectópico) en caso de embarazo
- una infección grave o septicemia (infección de la sangre). Esto es muy raro, pero podría ocurrir poco después de la inserción . Tener una septicemia significa que está muy enferma debido a una infección. Si no se trata, puede ser mortal
Le deben retirar Kyleena si la infección pélvica:
- reaparece varias veces
- es muy grave
- no desaparece con el tratamiento
Si Kyleena se sale
Kyleena puede ser expulsado de su sitio o caerse. Esto se debe a las contracciones musculares del útero durante la regla. Esto puede afectar hasta 1 de cada 10 personas, especialmente si:
- tiene sobrepeso en el momento de la inserción
- ha tenido reglas abundantes previas
Si el SLI se sale de su sitio, es posible que ya no actúe correctamente. Tendrá más riesgo de quedarse embarazada. Si se sale, ya no estará protegida frente a un embarazo.
Si el SLI no está en su sitio o se sale, puede sentir dolor o tener sangrados vaginales que son diferentes a los habituales. Asimismo, también puede salirse sin que se dé cuenta.
Kyleena normalmente reduce la cantidad de sangre que pierde durante la regla:
Cuanto más tiempo lo utilice, menos sangre perderá durante la regla. Esto significa que, si repentinamente vuelve a tener sangrados abundantes durante sus reglas, es posible que el SLI se haya salido. Consulte la sección 3 “Cómo comprobar por sí misma que Kyleena está en su sitio” para saber cómo comprobar si el SLI está en su sitio y qué hacer si sospecha que ya no está bien colocado.
Perforación
Puede ocurrir que Kyleena se introduzca en la pared del útero o la atraviese. Esto se denomina perforación. Una perforación suele producirse en el momento de la inserción . Una perforación no siempre duele, por lo que es posible que solo la note más tarde. Si ya no está colocado en su sitio debido a la perforación, ya no es eficaz frente al embarazo. En ese caso, un médico/profesional sanitario debe retirárselo lo antes posible. A veces, es necesaria una intervención quirúrgica.
Una perforación afecta hasta 1 de cada 1000personas. El riesgo de perforación es mayor (hasta 1 de cada 100personas) si:
- está en periodo de lactancia cuando se lo insertan
- ha tenido un bebé en los 9 meses anteriores a la inserción
Puede sufrir una perforación si:
- tiene dolor intenso parecido al dolor menstrual o más dolor del que esperaba
- sangra mucho por la vagina después de la inserción
- tiene dolor o sangrado que dura más de unas semanas
- tiene cambios repentinos en su patrón menstrual
- tiene dolor durante las relaciones sexuales
- ya no nota los hilos
Si cree que puede haber sufrido una perforación, póngase en contacto con un médico/profesional sanitario inmediatamente. Recuérdele que tiene insertado un SLI, especialmente si no fue la persona que se lo insertó.
Quiste ovárico
A veces se puede formar un pequeño saco lleno de líquido en el ovario . Este saco se conoce como quiste ovárico.
Los signos de un quiste ovárico pueden ser:
- dolor en la pelvis
- dolor o molestias durante las relaciones sexuales
Un quiste ovárico suele desaparecer por sí solo. Sin embargo, puede requerir asistencia médica o, más raramente, una intervención quirúrgica. Si cree que puede tener un quiste ovárico, póngase en contacto con su médico/profesional sanitario.
Problemas de salud mental
Algunas mujeres que utilizan anticonceptivos hormonales, incluido Kyleena, sufren depresión o estado de ánimo depresivo.
La depresión puede ser grave y, a veces, puede provocar pensamientos suicidas. Si tiene cambios de humor y síntomas depresivos, póngase en contacto con su médico/profesional sanitario lo antes posible. La depresión y el estado de ánimo depresivo pueden afectar hasta 1 de cada 100 personas que lo utilizan .
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico/profesional sanitario, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kyleena
No requiere condiciones especiales de conservación.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No abrir el blíster (envase de plástico que contine el SLI). Solo su médico o un profesional sanitario debe hacerlo.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Kyleena
El principio activoes el levonorgestrel. El sistema de liberación intrauterino contiene 19,5 mg de levonorgestrel.
Los demás componentesson:
- elastómero de polidimetilsiloxano
- sílice coloidal anhidra
- polietileno
- sulfato de bario
- polipropileno
- ftalocianina de cobre
- plata
Aspecto de Kyleena y contenido del envase
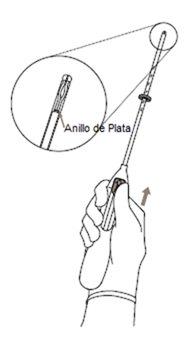
Sistema de liberación (SLI) para uso en el útero. Tiene forma de T y es blanco. La parte vertical es un pequeño depósito que contiene la hormona levonorgestrel. Hay dos hilos azules atados a un asa en el extremo inferior. Esto permite que un profesional sanitario retire el SLI. Hay también un anillo de plata situado cerca de los brazos horizontales. Su médico puede ver este anillo durante la ecografía.
Tamaño de envase:
- 1 x 1 sistema de liberación intrauterino.
- 5 x 1 sistema de liberación intrauterino.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
España
Responsable de la fabricación
Bayer Oy
Pansiontie 47
20210 Turku
Finlandia
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
- Austria, Bélgica, República Checa, Dinamarca, Estonia, Finlandia, Francia, Alemania, Islandia, Irlanda, Italia, Letonia, Lituania, Países Bajos, Noruega, Polonia, Portugal, Eslovenia, España, Suecia: Kyleena
Fecha de la última revisión de este prospecto:09/2024.
Otras fuentes de información
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el prospecto, cartonaje y tarjeta recordatorio para la paciente. También puede acceder a la misma información en la siguiente dirección de internet: https://cima.aemps.es/info/81418
[Inclusión a nivel nacional del código QR que le dirige a la ficha técnica]
Esta información está destinada únicamente a médicos/profesionales sanitarios:
INSTRUCCIONES DE INSERCIÓN
Kyleena 19,5 mg sistema de liberación intrauterino
levonorgestrel
Para inserción por un médico/profesional sanitario empleando una técnica aséptica.
Kyleena se suministra en un envase estéril dentro de un insertor que permite la manipulación con una sola mano. El envase no debe abrirse hasta que sea necesario para su inserción. No reesterilizar. En esta presentación, el SLI es para un solo uso. No utilizar si el blíster está dañado o abierto. No insertar después de la fecha de caducidad que aparece en la caja y en el blíster después de CAD.
La eliminación del medicamento no utilizado o del material de desecho se realizará de acuerdo con la normativa local.
El SLI se proporciona con una tarjeta recordatorio para la paciente dentro del envase. Complete la tarjeta recordatorio para la paciente y désela a la paciente después de la inserción.
Preparación para la inserción
- Examinar a la paciente para descartar contraindicaciones para la inserción (ver Ficha Técnica, sección 4.3 y sección 4.4, apartado “Exploración/consulta médica”).
- Insertar un espéculo, visualizar el cuello uterino y después limpiar meticulosamente el cuello uterino y la vagina con una solución antiséptica adecuada.
- Asistirse por un ayudante si es necesario.
- Sujetar el labio anterior del cuello uterino con un tenáculo u otras pinzas para estabilizar el útero. Si el útero está retrovertido, puede resultar más apropiado sujetar el labio posterior del cuello uterino. Se puede aplicar una tracción suave con las pinzas para enderezar el canal cervical. Las pinzas deben permanecer en su sitio y se debe aplicar una tracción contraria suave sobre el cuello uterino durante toda la intervención de inserción.
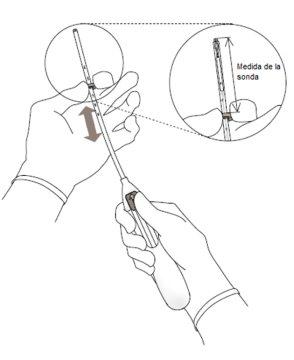
- Introducir una sonda uterina por el canal cervical hasta el fondo uterino para medir la profundidad y confirmar la dirección de la cavidad uterina y para descartar cualquier posibilidad de anomalía intrauterina (p. ej., tabique, fibromas submucosos) o de presencia de un anticonceptivo intrauterino insertado anteriormente que no haya sido retirado. Si se encuentran dificultades, considerar la dilatación del canal. Si es necesaria una dilatación cervical, valorar la utilización de analgésicos y/o de un bloqueo paracervical.
Inserción
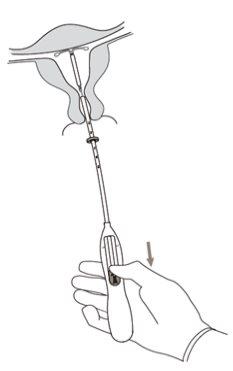
- Primero, abrir el envase estéril por completo (Figura 1). Después emplear una técnica aséptica y guantes estériles.
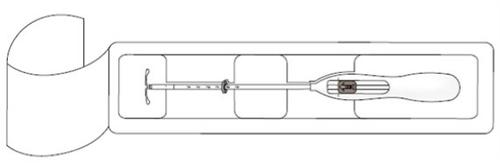







|
| Figura 2 |
¡IMPORTANTE!No tirar de la corredera hacia abajo porque esto puede liberar Kyleena prematuramente. Una vez liberado, no se puede volver a cargar.
|
| Figura 3 |
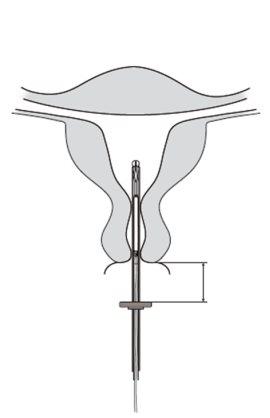
| 1,5 – 2,0 cm | Figura 4 |
¡IMPORTANTE!No forzar el insertor. Dilatar el canal cervical si es necesario.
|
| Figura 5 |
|
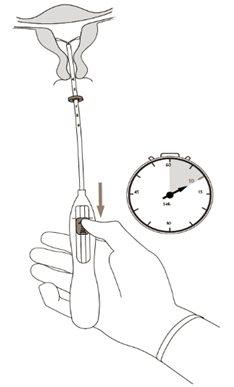
| Figura 6 |
|
| Figura 7 |
¡IMPORTANTE!Si se sospecha que el sistema no está en la posición correcta, comprobar su ubicación (p. ej., mediante una ecografía). Retirar el sistema si no está bien colocado dentro de la cavidad uterina. No debe reinsertarse un sistema retirado. |
Retirada/sustitución
Para la retirada/sustitución, consultar la Ficha Técnica de Kyleena.
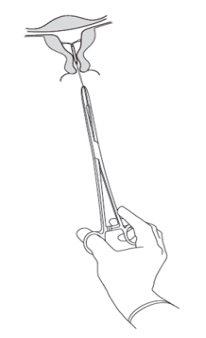
Se retira tirando de los hilos con unas pinzas (Figura 8). Se puede insertar un nuevo SLI inmediatamente después de la retirada. Después de la reirada, se debe examinar el sistema para asegurarse de que está intacto y se ha retirado por completo. |
| Figura 8 |
Inclusión a nivel nacional del código QR que le dirige a la Ficha Técnica La Ficha Técnica de Kyleena está disponible en la dirección de internet https://cima.aemps.es/info/81418 |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KYLEENA 19,5 MG SISTEMA DE LIBERACION INTRAUTERINOForma farmacéutica: DISPOSITIVO INTRAUTERINO, 13,5 mg levonorgestrelPrincipio activo: plastic IUD with progestogenFabricante: Bayer Hispania S.L.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 52 mg/ tasa de liberación inicial de 0,02 mg cada 24 hPrincipio activo: plastic IUD with progestogenFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: DISPOSITIVO INTRAUTERINO, 0,02 mg/24 hPrincipio activo: plastic IUD with progestogenFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para KYLEENA 19,5 MG SISTEMA DE LIBERACION INTRAUTERINO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KYLEENA 19,5 MG SISTEMA DE LIBERACION INTRAUTERINO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes