
KEVZARA 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar KEVZARA 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Kevzara 150 mg solución inyectable en pluma precargada
Kevzara 200 mg solución inyectable en pluma precargada
sarilumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Además de este prospecto, se le dará una tarjeta de información para el paciente, que contiene información de seguridad importante que necesita antes y durante el tratamiento con Kevzara.
Contenido del prospecto
- Qué es Kevzara y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kevzara
- Cómo usar Kevzara
- Posibles efectos adversos
- Conservación de Kevzara
- Contenido del envase e información adicional
1. Qué es Kevzara y para qué se utiliza
Qué es Kevzara
Kevzara contiene el principio activo sarilumab. Es un tipo de proteína denominada “anticuerpo monoclonal”.
Para qué se utiliza Kevzara
Kevzara se utiliza para tratar adultos con artritis reumatoide activa (AR) de moderada a grave si el tratamiento previo no ha funcionado bien o no se ha tolerado. Kevzara se puede utilizar solo o junto con un medicamento denominado MTX.
Le puede ayudar a:
- frenar el daño en las articulaciones
- mejorar su capacidad para realizar actividades diarias.
Kevzara se utiliza para tratar adultos con polimialgia reumática después de que se hayan utilizado corticosteroides y no hayan funcionado bien o si experimenta una recaída mientras disminuye la dosis de corticosteroides (reducción gradual). Kevzara se puede utilizar solo o junto con un medicamento llamado corticosteroide.
Cómo funciona Kevzara
- Kevzara se une al receptor de otra proteína denominada interleucina-6 (IL-6) y bloquea su acción.
- La IL-6 juega un papel principal en los síntomas de la AR como el dolor, la inflamación de las articulaciones, la rigidez por las mañanas y la fatiga.
2. Qué necesita saber antes de empezar a usar Kevzara
No use Kevzara:
- si es alérgico a sarilumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una infección activa grave.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero si:
- tiene cualquier infección o si contrae infecciones a menudo. Kevzara puede reducir la capacidad de su cuerpo para combatir la infección y esto quiere decir que puede hacerle más susceptible a contraer infecciones o a hacer que empeore su infección.
- tiene tuberculosis (TB), síntomas de TB (tos persistente, pérdida de peso, desgana, fiebre leve), o ha estado en contacto cercano con una persona con TB. Antes de iniciar un tratamiento con Kevzara, su médico le hará pruebas para la TB.
- ha tenido hepatitis viral u otra enfermedad del hígado. Antes de usar Kevzara, su médico le hará un análisis de sangre para revisar el funcionamiento de su hígado.
- ha tenido diverticulitis (una enfermedad del colon) o úlceras en su estómago o en los intestinos, o desarrolla síntomas como fiebre y dolor de estómago (dolor abdominal) que no desaparece.
- alguna vez ha tenido algún tipo de cáncer.
- ha sido vacunado recientemente o le van a vacunar.
Si alguno de los puntos anteriores le concierne (o si no está seguro), consulte a su médico, farmacéutico o enfermero antes de usar Kevzara.
Se deberá hacer análisis de sangre antes de que reciba Kevzara. También se deberá hacer análisis durante su tratamiento. Esto es para revisar si tiene un recuento bajo de células sanguíneas, problemas de hígado o cambios en sus niveles de colesterol.
Cada vez que reciba un nuevo envase de Kevzara, es importante que anote el nombre del medicamento, la fecha de administración y el número de lote (que aparece en el envase después de “Lote”) y guarde esta información en un lugar seguro.
Niños y adolescentes
La pluma precargada de Kevzara no se ha estudiado en niños a partir de 2 años de edad con AIJp y no está indicada para su uso en niños.
No se recomienda el uso de Kevzara en niños menores de 2 años de edad. Kevzara no se debe administrar a niños con AIJp que pesen menos de 10 kg.
Otros medicamentos y Kevzara
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento. Esto se debe a que Kevzara puede afectar a la manera en que actúan otros medicamentos. También otros medicamentos pueden afectar a la manera en que actúa Kevzara.
En particular, no use Kevzara e informe a su médico o farmacéutico si está usando:
- un grupo de medicamentos denominados “inhibidores de la Janus cinasa (JAK)” (utilizados para enfermedades como la artritis reumatoide y el cáncer)
- otros medicamentos biológicos usados en el tratamiento de la AR.
Si cualquiera de los puntos anteriores le concierne (o si no está seguro), consulte a su médico o farmacéutico.
Kevzara puede afectar a la forma de actuar de algunos medicamentos: esto quiere decir que puede ser necesario modificar la dosis de otros medicamentos. Si está usando alguno de los siguientes medicamentos, informe a su médico o farmacéutico antes de usar Kevzara:
- estatinas, usadas para reducir el nivel de colesterol
- anticonceptivos orales
- teofilina, usada para tratar el asma
- warfarina, usada para prevenir los coágulos de sangre.
Si cualquiera de los puntos anteriores le concierne (o si no está seguro), consulte a su médico o farmacéutico.
Embarazo y lactancia
Hable con su médico antes de utilizar Kevzara si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada.
- No use Kevzara si está embarazada a no ser que su médico se lo recomiende específicamente.
- Se desconocen los efectos de Kevzara sobre el feto.
- Su médico y usted deben decidir si usted debe recibir el tratamiento con Kevzara si está dando el pecho.
Conducción y uso de máquinas
No se espera que el uso de Kevzara afecte a su capacidad para conducir o utilizar máquinas. Sin embargo, si se siente cansado o siente malestar después de recibir el tratamiento con Kevzara, no debe conducir ni utilizar máquinas.
KEVZARA contiene polisorbato 20
Este medicamento contiene 2,28 mg de polisorbato 20 en cada 1,14 ml de solución inyectable equivalente a 2 mg/ml. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo usar Kevzara
El tratamiento se debe iniciar por un médico con experiencia en el diagnóstico y tratamiento de AR o polimialgia reumática. Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Pacientes adultos
La dosis recomendada es una inyección de 200 mg cada dos semanas.
- Su médico puede ajustar la dosis de su medicamento en base a los resultados de sus análisis de sangre.
Kevzara se administra como una inyección debajo de la piel (denominada inyección “subcutánea”).
Aprenda cómo usar la pluma precargada
- Su médico, farmacéutico o enfermero le enseñarán cómo inyectar Kevzara. Siguiendo estas instrucciones, Kevzara puede ser inyectado por usted mismo o administrado por un cuidador después de recibir una formación adecuada.
- Siga cuidadosamente las “Instrucciones de Uso” incluidas en el envase.
- Use la pluma precargada exactamente como se describe en las “Instrucciones de Uso”.
Si usa másKevzaradel que debe
Si ha utilizado más Kevzara del que debe, informe a su médico, farmacéutico o enfermero.
Si olvidó usar una dosis deKevzara
Si han transcurrido 3 días o menos desde la dosis olvidada:
- inyecte su dosis olvidada tan pronto como sea posible.
- entonces administre su siguiente dosis en el siguiente día programado.
Si han transcurrido 4 días o más, inyecte la siguiente dosis en el siguiente día programado. No se inyecte una dosis doble para compensar la inyección olvidada.
Si no está seguro de cuándo se debe inyectar su próxima dosis, pregunte a su médico, farmacéutico o enfermero para que le den instrucciones.
Si interrumpe el tratamiento conKevzara
No interrumpa el tratamiento con Kevzara sin comentarlo con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efecto adverso grave
Informe a su médicoinmediatamentesi piensa que tiene una infección(que puede afectar hasta 1 de cada 10 personas). Los síntomas pueden incluir fiebre, sudores o escalofríos.
Otros efectos adversos
Informe a su médico, farmacéutico o enfermero si observa alguno de los siguientes efectos adversos:
Adultos
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Recuentos bajos de células blancas de la sangre según los análisis de sangre
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- infecciones en sus senos nasales o en la garganta, congestión o goteo nasal y dolor de garganta (“infección del tracto respiratorio superior”)
- infección del tracto urinario
- herpes febril (“herpes oral”)
- recuentos bajos de plaquetas según los análisis de sangre
- colesterol alto, triglicéridos altos según los análisis de sangre
- pruebas anormales de la función hepática
- reacciones en el lugar de la inyección (incluyendo enrojecimiento y picor)
- inflamación del tejido profundo de la piel
- infección de los pulmones
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- diverticulitis (una enfermedad que afecta al intestino a menudo con dolor de estómago (abdominal), náusea y vómito, fiebre, y estreñimiento, o menos frecuentemente diarrea)
Raros(pueden afectar hasta 1 de cada 1 000 personas):
- perforación en el estómago o intestinos (un orificio que se desarrolla en la pared del intestino)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V*. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kevzara
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en la nevera (entre 2 °C y 8 °C).
- No congelar.
- Una vez fuera de la nevera, no conservar Kevzara a temperatura superior a 25 ºC.
- Escriba la fecha en la que retira la pluma de la nevera en el espacio provisto en el envase exterior.
- Use la pluma en los 14 días siguientes a sacarla de la nevera o de la bolsa isotérmica.
- Mantener la pluma en el envase original para proteger de la luz.
No utilice este medicamento si la solución en la pluma está turbia, decolorada o contiene partículas, o si alguna parte de la pluma precargada parece dañada.
Después de usar, ponga la pluma en un contenedor para objetos punzantes. Mantener siempre el contenedor fuera de la vista y del alcance de los niños. Pregunte a su médico, farmacéutico o enfermero cómo deshacerse del contenedor.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Kevzara
- El principio activo es sarilumab. Cada pluma precargada contiene 150 mg o 200 mg de sarilumab en 1,14 ml de solución.
- Los demás excipientes son arginina, histidina, polisorbato 20 (E 432), sacarosa y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Kevzara es una solución inyectable transparente, incolora a color amarillo pálido, que se presenta en una pluma precargada.
Cada pluma precargada contiene 1,14 ml de solución que proporciona una sola dosis. Kevzara está disponible en envases que contienen 1 o 2 plumas precargadas y envases múltiples que contienen 3 envases, cada uno con 2 plumas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Kevzara está disponible como plumas precargadas de 150 mg o 200 mg.
Titular de la autorización de comercialización
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
Francia
Responsable de la fabricación
Sanofi-Aventis Deutschland GmbH
Brüningstraße 50
Industriepark Höchst
65926 Frankfurt am Main
Alemania
Genzyme Ireland Ltd.
IDA Industrial Park
Old Kilmeaden Road
Waterford
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
--------------------------------------------------------------------------------------------------------------------
België/Belgique/Belgien Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Ceská republika Sanofi s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. +39. 02 39394275 |
Deutschland Sanofi-Aventis Deutschland GmbH Telefon: 0800 04 36 996 Telefon aus dem Ausland: +49 69 305 70 13 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Ελλ?δα Sanofi-Aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Österreich sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda Tel: +351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor ehf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536389 | Suomi/Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
Kevzara 200 mg solución inyectable en pluma precargada
sarilumab
Instrucciones de uso
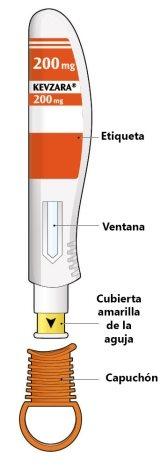
Las partes de la pluma precargada de Kevzara se muestran en este dibujo.
Información importante
Este dispositivo es una pluma precargada de una sola dosis (denominada “pluma” en estas instrucciones). Contiene 200 mg de Kevzara para inyección por debajo de la piel (inyección subcutánea) una vez cada dos semanas.
Solicite a su profesional sanitario que le muestre cómo usar de forma correcta la pluma antes de su primera inyección.
Qué debe hacer
- Lea todas las instrucciones detenidamente antes de utilizar una pluma.
- Compruebe que tiene el medicamento correcto y la dosis correcta.
- Guarde las plumas sin usar en el envase original y consérvelas en la nevera a una temperatura entre 2 °C y 8 °C.
- Guarde el envase en una bolsa isotérmica con un acumulador de frío cuando viaje.
- Deje que la pluma alcance la temperatura ambiente durante al menos 60 minutos antes de usarla.
- Use la pluma en un plazo de 14 días después de sacarla de la nevera o de la bolsa isotérmica.
- Mantenga la pluma fuera de la vista y del alcance de los niños.
Qué no debe hacer
- No use la pluma si esta ha sufrido daños o si falta el capuchón o no está sujeto.
- No quite el capuchón hasta que no esté preparado para la inyección.
- No presione ni toque la cubierta amarilla de la aguja con sus dedos.
- No trate de volver a ponerle el capuchón a la pluma.
- No reutilice la pluma.
- No congele ni caliente la pluma.
- Una vez se saca de la nevera, no conserve la pluma a temperatura superior a 25 °C.
- No exponga la pluma a la luz solar directa.
- No inyecte a través de la ropa.
Si tiene cualquier pregunta adicional, consulte con su médico, farmacéutico o enfermero.
Paso A: Preparación para la inyección
- Prepare todo el equipo que va a necesitar en una zona limpia y plana.
- Necesitará una toallita con alcohol, una bola de algodón o gasa, y un contenedor para objetos punzantes.
- Saque una pluma del envase cogiéndola por el medio del cuerpo de la pluma. Guarde la pluma restante en el envase en la nevera.
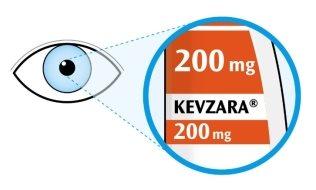
- Mire la etiqueta.
- Compruebe que tiene el medicamento correcto y la dosis correcta.
- Compruebe la fecha de caducidad (CAD), esta información se muestra en el lado de las plumas.
- Nouse la pluma si está caducada.
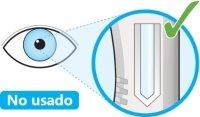
- Mire a la ventana.
- Compruebe que el líquido es transparente e incoloro a color amarillo pálido.
- Puede ver una burbuja de aire, esto es normal.
- Noproceda con la inyección si el líquido está turbio, decolorado o contiene partículas.
- Noutilice la pluma si la ventana es de color amarillo sólido.
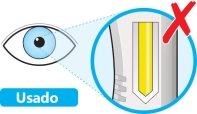
- Coloque la pluma sobre una zona plana y deje que alcance la temperatura ambiente (<25 ºC) durante al menos 60 minutos.
- Usar la pluma a temperatura ambiente puede hacer la inyección más cómoda.
- Nouse la pluma si esta ha estado fuera de la nevera más de 14 días.
- Nocaliente la pluma; deje que se atempere de forma natural.
- Noexponga la pluma a la luz solar directa.


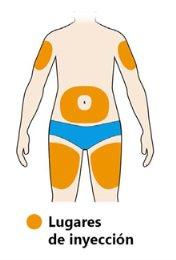
- Seleccione el lugar de la inyección.
- Puede inyectar en su muslo o vientre (abdomen) excepto en los 5 cm alrededor de su ombligo. Si alguien le pone la inyección, también puede elegir la parte exterior y superior del brazo.
- Cambie el lugar de la inyección cada vez que se inyecte.
- Norealice la inyección en la piel sensible, dañada ni con hematomas o cicatrices.
- Prepare el lugar de la inyección.
- Lávese las manos.
- Desinfecte la piel con una toallita con alcohol.
- Novuelva a tocar el lugar de la inyección antes de la inyección.
Paso B: Realice la inyección - Proceda con el Paso B solo tras completar el Paso A “Preparación para la inyección”
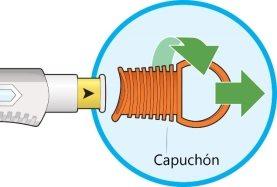
- Gire y retire el capuchón naranja.
- Noretire el capuchón hasta que esté preparado para la inyección.
- Nopresione ni toque la cubierta amarilla de la aguja con sus dedos.
- Novuelva a poner el capuchón.
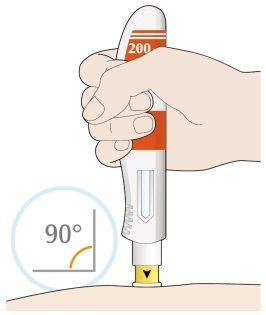
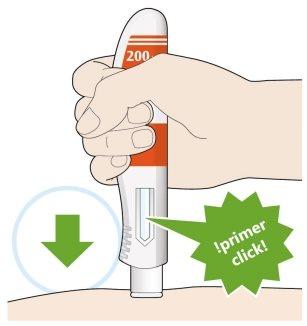
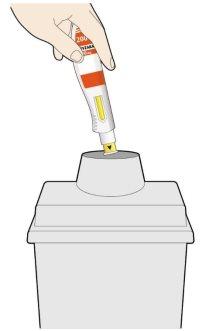
- Coloque la cubierta amarilla de la aguja sobre su piel con un ángulo aproximadamente de 90º.
- Asegúrese de que puede ver la ventana.
- Presione hacia abajo y mantenga la pluma firmemente contra su piel.
- Se producirá un “click” cuando comience la inyección.
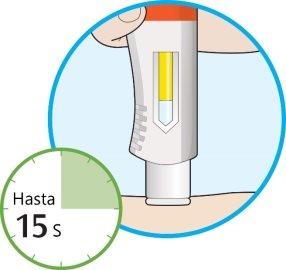
- Siga manteniendo la pluma firmemente contra su piel.
- La ventana empezará a cambiar de color a amarillo.
- La inyección puede tardar hasta 15 segundos (15 s).
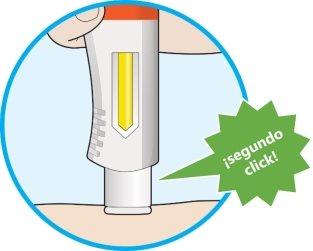
- Se producirá un segundo click. Compruebe que puede ver que toda la ventana se ha vuelto completamente amarilla antes de retirar la pluma.
- Si no oye el segundo click, debe seguir revisando para ver si la ventana se ha vuelto completamente amarilla.
- Si la ventana no se ha vuelto completamente amarilla, nose administre una segunda dosis sin hablar con su profesional sanitario.
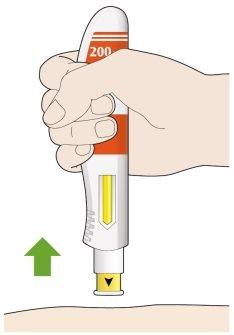
- Retire la pluma de su piel.
- Si ve algo de sangre, presione en el lugar de la inyección con una bola de algodón o una gasa.
- Nose frote la piel después de ponerse la inyección.
- Ponga la pluma que ha usado y el capuchón en un contenedor para objetos punzantes de forma inmediata tras su uso.
- Mantenga siempre el contenedor fuera de la vista y del alcance de los niños.
- Novuelva a poner el capuchón.
- Notire las plumas usadas a la basura.
- Notire el contenedor para objetos punzantes usado a la basura a no ser que la normativa local lo permita. Pregunte a su médico, farmacéutico o enfermero cómo deshacerse del contenedor.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KEVZARA 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: SarilumabFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: SarilumabFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: SarilumabFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para KEVZARA 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KEVZARA 200 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







