KESIMPTA 20 mg SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar KESIMPTA 20 mg SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Kesimpta 20 mg solución inyectable en pluma precargada
ofatumumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Kesimpta y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kesimpta
- Cómo usar Kesimpta
- Posibles efectos adversos
- Conservación de Kesimpta
- Contenido del envase e información adicional
1. Qué es Kesimpta y para qué se utiliza
Qué es Kesimpta
Kesimpta contiene el principio activo ofatumumab. Ofatumumab pertenece a un grupo de medicamentos conocidos como anticuerpos monoclonales.
Para qué se utiliza Kesimpta
Kesimpta se utiliza para tratar adultos con formas recurrentes de esclerosis múltiple (EMR).
Cómo funciona Kesimpta
Kesimpta funciona mediante la unión a una diana conocida como CD20 que está en la superficie de los linfocitos B. Los linfocitos B son un tipo de glóbulos blancos que forman parte del sistema inmunitario (las defensas del organismo). En la esclerosis múltiple, el sistema inmunitario ataca a la capa protectora de alrededor de las células nerviosas. Los linfocitos B están implicados en este proceso. Kesimpta se dirige a los linfocitos B y los elimina. De este modo reduce la posibilidad de un brote, alivia los síntomas y reduce la velocidad de progresión de la enfermedad.
2. Qué necesita saber antes de empezar a usar Kesimpta
No use Kesimpta
- si es alérgico al ofatumumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si le han diagnosticado problemas graves en su sistema inmunitario.
- si tiene una infección grave.
- si tiene cáncer.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Kesimpta
- Kesimpta puede provocar que el virus de la hepatitis B vuelva a activarse. Su médico le realizará un análisis de sangre para comprobar si tiene riesgo de infección por hepatitis B. Si el resultado muestra que ha tenido hepatitis B o que es portador del virus de la hepatitis B, su médico le pedirá que visite a un especialista.
- Antes de iniciar el tratamiento con Kesimpta, su médico puede comprobar su sistema inmunitario.
- Si tiene una infección, su médico puede decidir que no puede usar Kesimpta o que debe retrasar el tratamiento con Kesimpta hasta que la infección se haya resuelto.
- Su médico comprobará si necesita alguna vacuna antes de iniciar el tratamiento con Kesimpta. Si necesitara un tipo de vacuna conocida como vacuna viva o viva atenuada, ésta se debe administrar al menos 4 semanas antes de comenzar el tratamiento con Kesimpta. Otros tipos de vacunas se deben administrar al menos 2 semanas antes de comenzar el tratamiento con Kesimpta.
Mientras usa Kesimpta
Informe a su médico:
- si presenta una reacción general relacionada con la inyección o una reacción local en el lugar de la inyección. Estos son los efectos adversos más comunes del tratamiento con Kesimpta y se describen en la sección 4. Normalmente ocurren en el plazo de 24 horas después de haber inyectado Kesimpta, especialmente después de la primera inyección. La primera inyección se debe realizar bajo la guía de un profesional sanitario.
- si tiene una infección. Puede contraer infecciones con mayor facilidad o si ya presenta una infección, ésta podría empeorar. Esto se debe a que las células inmunitarias sobre las que actúa Kesimpta también ayudan a combatir infecciones. Las infecciones podrían ser graves y a veces incluso de amenaza para la vida.
- si tiene previsto vacunarse. Su médico le indicará si la vacuna que necesita es una vacuna viva, una vacuna viva atenuada u otro tipo de vacuna. Durante el tratamiento con Kesimpta no le deben administrar vacunas vivas o vivas atenuadas ya que puede provocarle una infección. Otros tipos de vacunas puede que no funcionen tan bien si se reciben durante el tratamiento con Kesimpta.
Informe a su médico inmediatamente si presenta alguno de los siguientes síntomas durante el tratamiento con Kesimpta, ya que podrían ser signos de una enfermedad grave:
- si presenta sarpullido, urticaria, dificultad en la respiración, hinchazón de la cara, párpados, labios, boca, lengua o garganta, opresión en el pecho, o se siente débil. Éstos pueden ser signos o síntomas de una reacción alérgica.
- si piensa que su esclerosis múltiple está empeorando (p. ej. presenta debilidad o cambios en la visión) o si advierte cualquier síntoma nuevo o inusual. Estos efectos pueden ser indicativos de un trastorno raro del cerebro conocido como leucoencefalopatía multifocal progresiva (LMP), que está provocada por la infección de un virus.
Niños y adolescentes
No administre este medicamento a niños y adolescentes menores de 18 años, debido a que Kesimpta aún no se ha estudiado en este grupo de edad.
Otros medicamentos y Kesimpta
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
En especial, informe a su médico o farmacéutico:
- si está tomando, ha tomado recientemente o pudiera tener que tomar medicamentos que afectan al sistema inmunitario. Esto se debe a que se podrían sumar los efectos sobre el sistema inmunitario.
- si tiene previsto vacunarse (ver anteriormente “Advertencias y Precauciones”).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Embarazo
Debe evitar quedarse embarazada mientras usa Kesimpta y durante los 6 meses posteriores a la fecha en que deje de usarlo.
Si es una mujer en edad fértil debe utilizar un método anticonceptivo efectivo durante el tratamiento y durante 6 meses después de interrumpir el tratamiento con Kesimpta. Consulte con su médico las opciones disponibles.
Si se queda embarazada o cree que podría estar embarazada durante el tratamiento o durante los 6 meses posteriores a la última dosis, informe a su médico inmediatamente. Su médico le informará de los riesgos potenciales de Kesimpta en el embarazo. Esto se debe a que Kesimpta puede reducir el número de células del sistema inmunitario (linfocitos B) tanto en la madre como en el feto. Su médico debe comunicar su embarazo a Novartis. Usted también puede comunicar su embarazo contactando con el representante local de Novartis (ver sección 6), además de contactar con su médico.
Lactancia
Kesimpta puede pasar a la leche materna. Consulte con su médico los beneficios y riesgos antes de dar el pecho mientras usa Kesimpta.
Vacunación de los bebés recién nacidos
Consulte a su médico o farmacéutico antes de vacunar a su bebé recién nacido si usted ha usado Kesimpta durante el embarazo (ver anteriormente “Advertencias y precauciones”).
Conducción y uso de máquinas
No es probable que Kesimpta afecte su capacidad para conducir y utilizar máquinas.
Kesimpta contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
Kesimpta contiene polisorbato80
Este medicamento contiene 0,08 mg de polisorbato 80 por dosis. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo usar Kesimpta
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Kesimpta se administra mediante inyección subcutánea (inyección debajo de la piel).
La primera inyección se debe realizar bajo la guía de un profesional sanitario.
Las plumas precargadas de Kesimpta son para un solo uso.
Puede consultar las instrucciones detalladas sobre cómo inyectar Kesimpta en el apartado “Instrucciones de uso de Kesimpta en Pluma Sensoready” al final de este prospecto.
‘Código QR a incluir’ + www.kesimpta.eu
Puede usar Kesimpta en cualquier momento del día (mañana, tarde o noche).
Cuánto Kesimpta y con qué frecuencia se administra
No exceda la dosis que su médico le ha recetado.
- La dosificación inicial es de 20 mg de Kesimpta que se administra el primer día de tratamiento (Semana 0) y al cabo de 1 semana y de 2 semanas (Semana 1 y Semana 2). Después de estas primeras 3 inyecciones, la semana siguiente no se debe administrar ninguna inyección (Semana 3).
- La dosis recomendada es de 20 mg de Kesimpta una vez al mes, comenzando la Semana 4.
Tiempo | Dosis |
Semana 0 (primer día del tratamiento) | 20 mg |
Semana 1 | 20 mg |
Semana 2 | 20 mg |
Semana 3 | Ninguna inyección |
Semana 4 | 20 mg |
Después, cada mes | 20 mg |
Durante cuánto tiempo usar Kesimpta
Continúe usando Kesimpta cada mes durante el tiempo que le indique su médico.
Su médico controlará periódicamente el estado de su enfermedad para comprobar si el tratamiento tiene el efecto deseado.
Si tiene cualquier duda sobre cuánto tiempo debe usar Kesimpta, pregunte a su médico, farmacéutico o enfermero.
Si usa más Kesimpta del que debe
Si se ha administrado demasiado Kesimpta, informe a su médico inmediatamente.
Si olvidó usar Kesimpta
Para obtener el beneficio completo de Kesimpta, es importante que se ponga cada inyección cuando le corresponda.
Si ha olvidado ponerse una inyección de Kesimpta, debe administrársela lo antes posible. No espere hasta la siguiente dosis prevista. Los tiempos de administración de las siguientes inyecciones se deben entonces calcular desde el día que se ha inyectado esta dosis y no en base al calendario original (ver también el apartado anterior “Cuánto Kesimpta y con qué frecuencia se administra”).
Si interrumpe el tratamiento con Kesimpta
No interrumpa el tratamiento con Kesimpta ni cambie su dosis sin comentarlo antes con su médico.
Algunos efectos adversos pueden estar relacionados con unos niveles bajos de linfocitos B en la sangre. Después de interrumpir el tratamiento con Kesimpta sus niveles de linfocitos B en sangre irán aumentando gradualmente hasta alcanzar niveles normales. Esto puede durar varios meses, durante los cuales puede experimentar todavía algunos de los efectos adversos descritos en este prospecto.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
A continuación se mencionan los efectos adversos de Kesimpta. Si alguno de estos efectos adversos le afecta de forma grave, informe a su médico, farmacéutico o enfermero.
Muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
- infecciones de las vías respiratorias altas, con síntomas como irritación de garganta y goteo nasal
- reacciones relacionadas con la inyección tales como fiebre, dolor de cabeza, dolor muscular, escalofríos y cansancio - éstas normalmente aparecen en las 24 horas siguientes a una inyección de Kesimpta y principalmente después de la primera inyección
- infecciones de las vías urinarias
- reacciones en el lugar de la inyección, tales como enrojecimiento, dolor, picor e hinchazón en el lugar de la inyección
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
- descenso en el nivel sanguíneo de una proteína conocida como inmunoglobulina M, que ayuda a proteger frente a infecciones
- herpes oral
- náuseas, vómitos (se han comunicado asociados con reacciones relacionadas con la inyección)
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- reacciones alérgicas, con síntomas como sarpullido, urticaria, dificultad en la respiración, hinchazón de la cara, párpados, labios, boca, lengua o garganta, opresión en el pecho, o sensación de debilidad
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kesimpta
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja después de CAD y en la etiqueta después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar la(s) pluma(s) precargada(s) en el embalaje exterior para protegerla(s) de la luz. Conservar en nevera (entre 2 °C y 8 °C). No congelar.
En caso necesario, Kesimpta se puede dejar fuera de la nevera durante un periodo único de hasta 7 días a temperatura ambiente (no superior a 30 °C). Si no se utiliza durante dicho periodo, Kesimpta puede volver a ponerse en la nevera durante un máximo de 7 días.
No utilice este medicamento si observa que la solución contiene partículas visibles o está turbia.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Kesimpta
- El principio activo es ofatumumab. Cada pluma precargada contiene 20 mg de ofatumumab.
- Los demás componentes son L-arginina, acetato de sodio trihidrato, cloruro de sodio, polisorbato 80 (E 433), edetato disódico dihidrato, ácido clorhídrico (para ajuste del pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Kesimpta solución inyectable es una solución entre transparente y ligeramente opalescente, y entre incolora y amarilla-ligeramente amarronada.
Kesimpta está disponible en envases unitarios que contienen 1 Pluma Sensoready precargada y en envases múltiples que se componen de 3 cajas, con 1 Pluma Sensoready precargada en cada una.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsable de la fabricación
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Alemania
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλ?δα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κ?προς Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.




Instrucciones de uso de Kesimpta en pluma Sensoready
Es importante que entienda y siga estas instrucciones de uso antes de inyectarse Kesimpta. Si tiene alguna duda consulte a su médico, farmacéutico o enfermero antes de usar Kesimpta por primera vez.
Recuerde:
- No usela pluma si el precinto de la caja o el precinto de la pluma están rotos. Conserve la pluma dentro de la caja precintada hasta que esté listo para utilizarla.
- No agitela pluma.
- Si se cae la pluma, no la utilicesi parece que está dañada, o si la pluma cayó sin tener el tapón colocado.
- Deseche la pluma usada inmediatamente después de utilizarla. No reutilice una pluma. Ver el apartado “¿Cómo debo eliminar la pluma Sensoready de Kesimpta utilizada?” al final de estas Instrucciones de Uso.
¿Cómo debo conservar Kesimpta?
- Conserve la caja de la pluma en la nevera entre 2 °C y 8 °C.
- Conserve la pluma en el embalaje original para protegerla de la luz, hasta que esté listo para usarla.
- No congelela pluma.
Mantener Kesimpta fuera de la vista y del alcance de los niños.
Partes de la pluma Sensoready de Kesimpta (ver Imagen A):
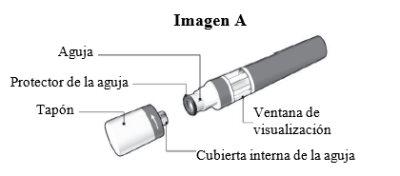
La pluma Sensoready de Kesimpta se muestra con el tapón quitado. No quite el tapón hasta que esté listo para la inyección.
Qué necesita para la inyección:
Incluido en la caja:
|
|
No incluido en la caja (ver Imagen C):
|
|
Ver el apartado “¿Cómo debo eliminar la pluma Sensoready de Kesimpta utilizada?” al final de estas
Instrucciones de Uso.
Antes de la inyección:
Saque la pluma de la nevera de 15 a 30 minutos antes de la inyecciónpara que alcance la temperatura ambiente.
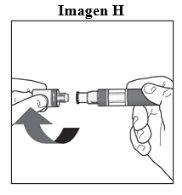
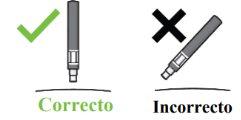
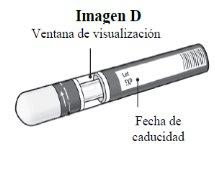
Paso 1. Comprobaciones de seguridad importantes antes de realizar la inyección (ver Imagen D):
No usela pluma si el líquido contiene partículas visibles o está turbio. Puede que vea una burbuja de aire pequeña, lo cual es normal.
Contacte con el farmacéutico o profesional sanitario si la pluma no cumple con alguna de estas comprobaciones. |
|
|
|
Su inyección Paso 4. Quite el tapón:
Puede que observe alguna gota de medicamento salir de la aguja. Es normal. |
|
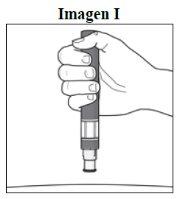
Paso 5. Sujete la pluma:
|
|
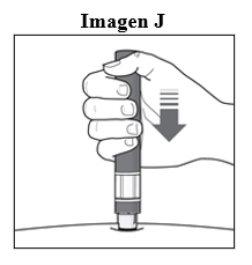
Paso 6. Inicie la inyección:
|
|
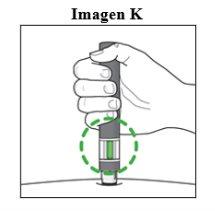
Paso 7. Finalice la inyección:
|
|
Importante: Durante la inyecciónoirá 2 clics intensos:
Debe mantener la pluma firmemente presionada contra la piel hasta que el indicador verdellene la ventana y haya dejado de moverse. |
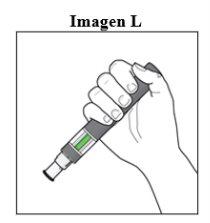
Después de la inyección:
|
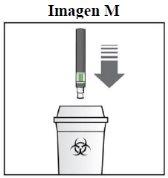
¿Cómo debo eliminar la pluma Sensoready de Kesimpta utilizada?
Paso 8. Elimine la pluma Sensoread de Kesimpta:
Mantenga el contenedor de eliminación de objetos punzantes fuera del alcance de los niños. |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KESIMPTA 20 mg SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para KESIMPTA 20 mg SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KESIMPTA 20 mg SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






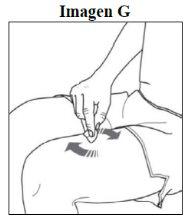
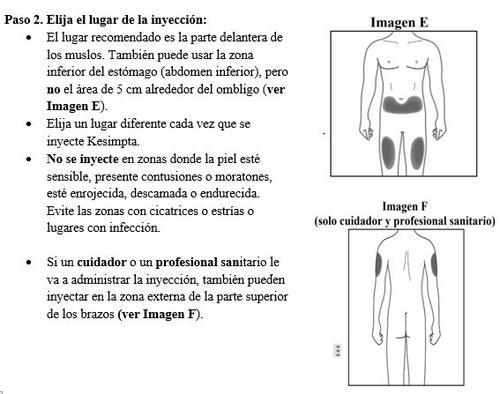
 Paso 3. Limpie el lugar de la inyección:
Paso 3. Limpie el lugar de la inyección: