
IDACIO 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar IDACIO 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Idacio 40 mg solución inyectable en pluma precargada
adalimumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Su médico le dará una tarjeta de información para el paciente, que contiene información de seguridad importante que necesita conocer antes y durante el tratamiento con Idacio.
Conserve esta tarjeta de información para el paciente durante su tratamiento y los 4 meses posteriores a su última inyección (o de su hijo) de Idacio.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Idacio y para qué se utiliza
- Qué necesita saber antes de empezar a usar Idacio
- Cómo usar Idacio
- Posibles efectos adversos
- Conservación de Idacio
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es Idacio y para qué se utiliza
Idacio contiene como sustancia activa adalimumab, un medicamento que actúa sobre el sistema inmunológico (defensa) de su cuerpo.
Idacio está indicado en el tratamiento de las siguientes enfermedades inflamatorias:
- artritis reumatoide,
- artritis idiopática juvenil poliarticular,
- artritis asociada a entesitis,
- espondilitis anquilosante,
- espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante,
- artritis psoriásica,
- psoriasis,
- hidradenitis supurativa,
- enfermedad de Crohn,
- colitis ulcerosa y
- uveítis no infecciosa.
La sustancia activa de Idacio, adalimumab, es un anticuerpo monoclonal. Los anticuerpos
monoclonales son proteínas que atacan a una diana específica del cuerpo.
La diana de adalimumab es otra proteína llamada factor de necrosis tumoral (TNFα), que se encuentra en niveles elevados en las enfermedades inflamatorias descritas arriba. Mediante el ataque al TNFα, Idacio bloquea su acción y reduce el proceso de inflamación en esas enfermedades.
Artritis reumatoide
La artritis reumatoide es una enfermedad inflamatoria de las articulaciones.
Idacio se utiliza para tratar la artritis reumatoide en adultos. Si usted padece artritis reumatoide activa moderada a grave, puede que se le administren antes otros medicamentos modificadores de la enfermedad tales como metotrexato. Si el efecto de estos medicamentos no es suficiente, se le administrará Idacio para tratar su artritis reumatoide.
Idacio también puede usarse en el tratamiento de la artritis reumatoide grave, activa y progresiva sin tratamiento previo con metotrexato.
Idacio puede reducir el daño de los cartílagos y huesos de las articulaciones producido por la enfermedad y mejora el rendimiento físico.
Habitualmente Idacio se usa junto con metotrexato. Si su médico considera que el metotrexato no es apropiado, Idacio puede administrarse solo.
Artritis idiopática juvenil poliarticular y artritis asociada a entesitis
La artritis idiopática juvenil poliarticular y la artritis asociada a entesitis son enfermedades inflamatorias de las articulaciones que suelen aparecer por primera vez en la infancia.
Idacio se utiliza para tratar la artritis idiopática juvenil poliarticular en niños y adolescentes de edades comprendidas entre los 2 y los 17 años y la artritis asociada a entesitis en niños entre 6 y 17 años. Los pacientes pueden haber recibido primero otros fármacos modificadores de la enfermedad, como metotrexato. Si la acción de estos medicamentos no es suficiente, los pacientes recibirán Idacio para tratar su artritis idiopática poliarticular o artritis asociada a entesitis.
Espondilitis anquilosante y espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante
La espondilitis anquilosante y la espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante son enfermedades inflamatorias que afectan a la columna vertebral.
Idacio se utiliza en adultos para tratar estas enfermedades. Si tiene espondilitis anquilosante o espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante, será tratado primero con otros medicamentos. Si la acción de estos medicamentos no es suficiente, recibirá Idacio para reducir los signos y síntomas de su enfermedad.
Artritis psoriásica
La artritis psoriásica es una enfermedad inflamatoria de las articulaciones asociada con la psoriasis.
Idacio se utiliza para tratar la artritis psoriásica en adultos. Idacio puede reducir el daño articular que produce la enfermedad en el cartílago y en el hueso y mejorar el rendimiento físico.
Psoriasis en placas en adultos y niños
La psoriasis en placas es una enfermedad inflamatoria de la piel que causa áreas enrojecidas, escamosas, con costras y cubiertas por escamas plateadas. La psoriasis en placas también puede afectar las uñas, provocando que se deterioren, se engrosen y se levanten del lecho de la uña, lo cual puede ser doloroso. Se cree que la psoriasis está causada por un defecto en el sistema inmune del cuerpo que lleva a un incremento en la producción de células de la piel.
Idacio se utiliza para tratar la psoriasis en placas de moderada a grave en adultos. Idacio también se utiliza para tratar la psoriasis en placas grave en niños y adolescentes entre 4 y 17 años de edad que no hayan respondido o no sean buenos candidatos para el uso de medicamentos aplicados sobre la piel ni para tratamientos con luz ultravioleta.
Hidradenitis supurativa en adultos y adolescentes
La hidradenitis supurativa (a veces denominada acné inverso) es una enfermedad inflamatoria de la piel de larga duración y a menudo dolorosa. Los síntomas pueden incluir nódulos sensibles (bultos) y abscesos (forúnculos) que pueden secretar pus. Normalmente afecta a áreas específicas de la piel, como debajo del pecho, de las axilas, zona interior de los muslos, ingle y nalgas. También puede haber cicatrices en las áreas afectadas.
Idacio se utiliza para tratar la hidradenitis supurativa en adultos y adolescentes a partir de 12 años. Idacio puede reducir el número de nódulos y abscesos, y el dolor que normalmente va asociado a esta enfermedad. Puede haber recibido otros medicamentos previamente. Si la acción de estos medicamentos no es suficiente, recibirá Idacio.
Enfermedad de Crohn en adultos y niños
La enfermedad de Crohn es una enfermedad inflamatoria del intestino.
Idacio se utiliza para tratar la enfermedad de Crohn en adultos y niños de edades comprendidas entre los 6 y los 17 años. Si padece enfermedad de Crohn será tratado primero con otros medicamentos. Si no responde suficientemente a estos medicamentos, recibirá Idacio para reducir los signos y síntomas de la enfermedad de Crohn.
Colitis ulcerosa en adultos y niños
La colitis ulcerosa es una enfermedad inflamatoria del intestino grueso.
Idacio se utiliza para tratar la colitis ulcerosa de moderada a grave en adultos y niños de 6 a 17 años. Si usted sufre colitis ulcerosa es posible que primero le receten otros medicamentos. Si la acción de estos medicamentos no resulta suficiente, le recetarán Idacio para reducir los signos y los síntomas de su enfermedad.
Uveítis no infecciosa en adultos y niños
La uveítis no infecciosa es una enfermedad inflamatoria que afecta a ciertas partes del ojo.
- Adultos con uveítis no infecciosa con inflamación que afecta a la parte posterior del ojo.
- Niños desde los 2 años de edad con uveítis crónica no infecciosa con inflamación que afecta a la parte frontal del ojo.
La inflamación puede dar lugar a una disminución de la visión y/o la presencia de motas en el ojo (puntos negros o líneas delgadas que se mueven a lo largo del campo de visión). Idacio actúa reduciendo esta inflamación.
Idacio se utiliza para tratar:
- adultos con uveítis no infecciosa con inflamación que afecta a la parte posterior del ojo.
- niños desde los 2 años de edad con uveítis crónica no infecciosa con inflamación que afecta a la parte frontal del ojo.
2. Qué necesita saber antes de empezar a usar Idacio
No use Idacio
- si es alérgico a adalimumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si padece una infección grave, incluida tuberculosis, sepsis (infección de la sangre) u otras infecciones oportunistas (infecciones inusuales asociadas con un sistema inmunitario debilitado). En caso de tener síntomas de cualquier infección, por ejemplo: fiebre, heridas, cansancio, problemas dentales, es importante que informe a su médico (ver “Advertencias y precauciones”).
- si padece insuficiencia cardiaca moderada o grave. Es importante que le diga a su médico si ha tenido o tiene algún problema cardiaco serio (ver “Advertencias y precauciones”).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Idacio.
Reacción alérgica
- Si presenta una reacción alérgica con síntomas como opresión en el pecho, dificultad para respirar, mareo, hinchazón o sarpullido, interrumpa la administración de Idacio y póngase en contacto con su médico inmediatamente ya que, en casos raros, estas reacciones pueden poner en peligro la vida.
Infección
- Si padece cualquier infección, incluyendo las crónicas o las localizadas (por ejemplo: una úlcera en la pierna), consulte a su médico antes de comenzar el tratamiento con Idacio. Si no está seguro, póngase en contacto con su médico.
- Con el tratamiento con Idacio podría contraer infecciones con más facilidad. Este riesgo puede ser mayor si presenta una función pulmonar reducida. Estas infecciones pueden ser más graves e incluyen tuberculosis, infecciones causadas por virus, hongos, parásitos o bacterias u otros organismos infecciosos inusuales y sepsis (infección de la sangre). En casos raros, estas infecciones pueden poner en peligro su vida. Por esta razón, es importante que en el caso de que tenga síntomas como fiebre, heridas, cansancio o problemas dentales, se lo diga a su médico. Su médico le puede recomendar que interrumpa temporalmente el tratamiento con Idacio.
Tuberculosis (TB)
- Dado que se han descrito casos de tuberculosis en pacientes en tratamiento con Idacio, su médico le examinará en busca de signos o síntomas de tuberculosis antes de comenzar su tratamiento con Idacio. Esto incluirá la realización de una evaluación médica minuciosa, incluyendo su historia médica y las pruebas de diagnóstico (por ejemplo, radiografía de tórax y la prueba de la tuberculina). La realización y resultados de estas pruebas debe anotarse en su tarjeta de información para el paciente. Es muy importante que informe a su médico en caso de haber padecido tuberculosis o haber estado en contacto con un paciente de tuberculosis. Se puede desarrollar tuberculosis durante el tratamiento incluso si usted ha recibido el tratamiento preventivo para la tuberculosis. Si apareciesen síntomas de tuberculosis (tos persistente, pérdida de peso, malestar general, febrícula) o de cualquier otra infección durante o una vez finalizado el tratamiento, póngase en contacto inmediatamente con su médico.
Infección por viaje/recurrente
- Informe a su médico si ha vivido o viajado por regiones en las que infecciones fúngicas como histoplasmosis, coccidiomicosis o blastomicosis son frecuentes.
- Informe a su médico si tiene antecedentes de infecciones recurrentes u otras patologías o factores que aumenten el riesgo de infecciones.
Virus de la Hepatitis B
- Informe a su médico si es usted portador del virus de la hepatitis B (VHB), si tiene infección activa con VHB o si piensa que podría correr riesgo de contraer el VHB. Su médico le debe realizar un análisis para el VHB. Adalimumab puede reactivar la infección por VHB en personas portadoras de este virus. En casos raros, especialmente si está tomando otros medicamentos que suprimen el sistema inmune, la reactivación de la infección por VHB puede poner en peligro su vida.
Paciente mayor de 65 años
- Si tiene más de 65 años puede ser más susceptible de padecer infecciones mientras está en tratamiento con Idacio. Tanto usted como su médico deben prestar atención especial a la aparición de signos de infección mientras esté siendo tratado con Idacio. Es importante informar a su médico si tiene síntomas de infecciones, como fiebre, heridas, sensación de cansancio o problemas dentales.
Intervenciones quirúrgicas o dentales
- Si le van a realizar una intervención quirúrgica o dental, informe a su médico de que está usando Idacio. Su médico le puede recomendar que interrumpa temporalmente el tratamiento con Idacio.
Enfermedad desmielinizante
- Si padece o desarrolla una enfermedad desmielinizante (una enfermedad que afecta a la capa aislante que rodea los nervios, como la esclerosis múltiple), su médico decidirá si debe ser tratado o continuar en tratamiento con Idacio. Informe inmediatamente a su médico si siente síntomas tales como cambios en la visión, debilidad en brazos o piernas o entumecimiento u hormigueo en cualquier parte del cuerpo.
Vacunas
- Ciertas vacunas contienen formas vivas pero debilitadas de bacterias o virus que causan enfermedades y no se deben administrar durante el tratamiento con Idacio en el caso de que estas vacunas causen infecciones. Consulte con su médico antes de la administración de cualquier tipo de vacuna. Si es posible, se recomienda que los niños reciban todas las vacunas establecidas en los calendarios de vacunación para su edad antes de iniciar el tratamiento con Idacio. Si recibe Idacio mientras está embarazada, su hijo puede tener un riesgo mayor de sufrir infecciones durante unos 5 meses siguientes a la última dosis que haya recibido de Idacio durante su embarazo. Es importante que informe al médico de su hijo y a otros profesionales sanitarios sobre su uso de Idacio durante el embarazo, para que ellos puedan decidir si su hijo debe recibir alguna vacuna.
Insuficiencia cardiaca
- Es importante que informe a su médico si ha padecido o padece problemas graves de corazón. Si padece insuficiencia cardiaca leve y está en tratamiento con Idacio, su médico debe hacerle un seguimiento continuo de su insuficiencia cardiaca. En caso de que aparezcan nuevos síntomas de insuficiencia cardíaca o empeoren los actuales (por ejemplo: dificultad al respirar, o hinchazón de los pies), debe ponerse en contacto con su médico inmediatamente.
Fiebre, cardenales, hemorragia o aspecto pálido
- En algunos pacientes, el organismo puede ser incapaz de producir un número suficiente del tipo de células sanguíneas que luchan contra las infecciones (glóbulos blancos) o de las que contribuyen a parar las hemorragias (plaquetas). Si tiene fiebre persistente, o sufre cardenales o sangra muy fácilmente o está muy pálido, consulte enseguida a su médico. Su médico puede decidir la interrupción del tratamiento.
Cáncer
- En muy raras ocasiones se han dado casos de ciertos tipos de cáncer en niños y adultos tratados con adalimumab u otros agentes que bloquean el TNFα. Las personas con artritis reumatoide de grados más graves y que padezcan la enfermedad desde hace mucho tiempo pueden tener un riesgo mayor que la media de desarrollar un linfoma y leucemia (cánceres que afectan a las células sanguíneas y a la médula ósea). Si está en tratamiento con Idacio el riesgo de padecer linfoma, leucemia y otros tipos de cáncer puede incrementarse. Se ha observado, en raras ocasiones, un tipo de linfoma específico y grave en pacientes en tratamiento con adalimumab. Algunos de estos pacientes recibían tratamiento también con azatioprina o mercaptopurina.
Informe a su médico si está tomando azatioprina o 6-mercaptopurina con adalimumab.
- Además, se han observado casos de cáncer de piel (tipo no melanoma) en pacientes que usan adalimumab. Avise a su médico si durante o después del tratamiento aparecen nuevas zonas dañadas en su piel o si las marcas o zonas dañadas existentes cambian de apariencia.
- Se han registrado cánceres, diferentes del linfoma, en pacientes con una determinada enfermedad pulmonar, denominada enfermedad pulmonar obstructiva crónica (EPOC), tratados con otro agente bloqueante del TNFα. Si tiene EPOC, o fuma mucho, debe consultar a su médico si el tratamiento con un bloqueante del TNFα es adecuado en su caso.
Síndrome similar al lupus
- En raras ocasiones el tratamiento con Idacio podría dar lugar a un síndrome similar al lupus. Contacte con su médico si tiene síntomas como erupción persistente sin explicación, fiebre, dolor de las articulaciones o cansancio.
Niños y adolescentes
- Vacunación: si es posible su hijo debe ponerse al día con todas las vacunas antes de usar Idacio.
- No administre Idacio a niños menores de 2 años con artritis idiopática juvenil poliarticular.
- No use la jeringa precargada de 40 mg o la pluma precargada de 40 mg si se recomiendan dosis distintas de 40 mg.
Otros medicamentos e Idacio
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Idacio se puede tomar junto con metotrexato o con ciertos medicamentos antirreumáticos modificadores de la enfermedad (sulfasalazina, hidroxicloroquina, leflunomida y preparaciones inyectables a base de sales de oro), corticoesteroides o medicamentos para el dolor, incluidos los antiinflamatorios no esteroideos (AINEs).
No debe utilizar Idacio junto con medicamentos cuyos principios activos sean anakinra o abatacept debido a un incremento del riesgo de infecciones graves. La combinación de adalimumab y otros antagonistas de TNFα y anakinra o abatacept no se recomienda debido al posible aumento del riesgo de infecciones, incluidas infecciones graves y otras posibles interacciones farmacológicas. Si tiene alguna duda, consulte a su médico.
Embarazo y lactancia
- Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Se le recomienda evitar quedarse embarazada y debe hacer uso de métodos anticonceptivos adecuados durante el tratamiento con Idacio y continuar con su uso durante al menos 5 meses después de la última administración de Idacio. Si se queda embarazada, deberá acudir a su médico.
- Idacio se debe usar durante el embarazo solo si es necesario.
- Según un estudio en embarazo, no hubo un mayor riesgo de defectos congénitos cuando la madre había recibido tratamiento con adalimumab durante el embarazo comparado con las madres con la misma enfermedad que no recibieron tratamiento con adalimumab.
- Idacio se puede usar durante la lactancia.
- Si utiliza Idacio mientras está embarazada, su hijo puede tener un riesgo más alto de contraer una infección.
- Es importante que informe al pediatra y a otros profesionales sanitarios sobre el uso de Idacio durante el embarazo antes de que el bebé reciba ninguna vacuna (para más información sobre vacunas ver sección “Vacunación”).
Conducción y uso de máquinas
La influencia de Idacio sobre la capacidad para conducir, montar en bicicleta o utilizar máquinas es pequeña. Puede producirse sensación de que la habitación da vueltas (vértigo) y alteraciones de la visión después de utilizar Idacio.
Idacio contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1mmol) por dosis de 0,8 ml, esto es, esencialmente “exento de sodio”.
3. Cómo usar Idacio
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Idacio se inyecta bajo la piel (vía subcutánea). Los pacientes que necesiten una dosis menor de 40 mg deben usar la presentación del vial de 40 mg de Idacio.
La dosis recomendada de Idacio en cada uno de los usos aprobados se muestran en la siguiente tabla.
Artritis reumatoide, artritis psoriásica, espondilitis anquilosante o espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Adultos | 40 mg en semanas alternas | En artritis reumatoide, el tratamiento con metotrexato se mantiene junto con Idacio. Si su médico decide que el metotrexato no es adecuado, Idacio puede administrarse solo. Si tiene artritis reumatoide y no recibe metotrexato junto con Idacio, su médico puede decidir administrar 40 mg semanalmente u 80 mg en semanas alternas. |
Artritis idiopática juvenil poliarticular | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Niños, adolescentes y adultos desde los 2 años que pesen 30 kg o más | 40 mg en semanas alternas | No aplica |
Niños y adolescentes desde 2 años que pesen entre 10 kg y menos de 30 kg | 20 mg en semanas alternas | No aplica |
Artritis relacionada con entesitis | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Niños, adolescentes y adultos desde 6 años que pesen 30 kg o más | 40 mg en semanas alternas | No aplica |
Niños y adolescentes desde 6 años que pesen entre 15 kg y menos de 30 kg | 20 mg en semanas alternas | No aplica |
Psoriasis en placa | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Adultos | Dosis inicial de 80 mg (dos inyecciones de 40 mg en un día), seguida de 40 mg en semanas alternas, comenzando una semana después de la dosis inicial. Debe continuar inyectándose Idacio durante tanto tiempo como le haya indicado su médico. | Dependiendo de su respuesta, su médico puede aumentar la dosis a 40 mg semanales u 80 mg en semanas alternas. |
Niños y adolescentes desde 4 a 17 años que pesen 30 kg o más | Dosis inicial de 40 mg, seguida de 40 mg una semana después. A partir de entonces, la dosis habitual es de 40 mg en semanas alternas. | No aplica |
Niños y adolescentes desde 4 a 17 años que pesen entre 15 kg de peso y menos de 30 kg | Dosis inicial de 20 mg, seguida de 20 mg una semana después. A partir de entonces, la dosis habitual es de 20 mg en semanas alternas. | No aplica |
Hidradenitis supurativa | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Adultos | Dosis inicial de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg por día en dos días consecutivos), seguido de una dosis de 80 mg (como dos inyecciones de 40 mg en el mismo día) dos semanas después. Después de dos semanas más, continúe con una dosis de 40 mg cada semana u 80 mg en semanas alternas, según se lo haya indicado su médico. | Se recomienda que utilice a diario un líquido antiséptico en las zonas afectadas. |
Adolescentes desde 12 hasta 17 años que pesen 30 kg o más | Dosis inicial de 80 mg (como dos inyecciones de 40 mg en un día), seguidas de 40 mg en semanas alternas comenzando una semana después. | Dependiendo de su respuesta, su médico puede incrementar la dosis a 40 mg semanales u 80 mg en semanas alternas. Se recomienda que utilice a diario un líquido antiséptico en las zonas afectadas. |
Enfermedad de Crohn | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Niños, adolescentes y adultos desde 6 años que pesen 40 kg o más | Dosis inicial de 80 mg (como dos inyecciones de 40 mg en un día), seguidas de 40 mg dos semanas después. Si se requiere una respuesta más rápida, su médico puede prescribir una dosis inicial de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg por día durante dos días consecutivos) seguida de 80 mg (como dos inyecciones de 40 mg en un día) dos semanas después. A partir de entonces, la dosis habitual es de 40 mg en semanas alternas. | Dependiendo de su respuesta, su médico puede incrementar la dosis a 40 mg semanales u 80 mg en semanas alternas. |
Niños y adolescentes desde 6 a 17 años que pesen menos de 40 kg | Dosis inicial de 40 mg, seguida de 20 mg dos semanas después. Si se requiere una respuesta más rápida, su médico puede prescribirle una primera dosis de 80 mg (dos inyecciones de 40 mg en un día), seguidas de 40 mg dos semanas después. A partir de entonces, la dosis habitual es de 20 mg en semanas alternas. | Dependiendo de su respuesta, su médico puede incrementar la frecuencia de la dosis a 20 mg semanales. |
Colitis ulcerosa | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Adultos | La dosis inicial es de 160 mg (cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg por día en dos días consecutivos) seguida de 80 mg (dos inyecciones de 40 mg en un día). A partir de entonces, la dosis habitual es de 40 mg en semanas alternas. | Dependiendo de su respuesta, su médico puede incrementar la dosis a 40 mg semanales u 80 mg cada dos semanas. |
Niños y adolescentes de 6 a 17 años de edad con un peso de 40 kg o superior | Primera dosis de 160 mg (cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg por día en dos días consecutivos), seguida de 80 mg (dos inyecciones de 40 mg en un día) dos semanas después. A partir de entonces, la dosis habitual es de 80 mg en semanas alternas. | Debe continuar utilizando la dosis habitual de 80 mg en semanas alternas, incluso después de cumplir los 18 años. |
Niños y adolescentes de 6 a 17 años de edad con un peso inferior a 40 kg | Primera dosis de 80 mg (dos inyecciones de 40 mg en un día), seguida de 40 mg (una inyección de 40 mg) dos semanas después. A partir de entonces, la dosis habitual es de 40 mg en semanas alternas. | Debe continuar utilizando la dosis habitual, 40 mg en semanas alternas, incluso después de cumplir los 18 años. |
Uveítis no infecciosa | ||
Edad o peso corporal | Cantidad y frecuencia de administración | Notas |
Adultos | Dosis inicial de 80 mg (dos inyecciones en un día), seguida de 40 mg en semanas alternas comenzando una semana después de la dosis inicial. Debe continuar inyectándose Idacio durante el tiempo que le haya indicado su médico. | Se puede continuar el tratamiento con corticoesteroides u otros medicamentos que afectan el sistema inmune mientras se usa Idacio. Idacio también se puede administrar sólo. |
Niños y adolescentes desde 2 años que pesen menos de 30 kg | 20 mg en semanas alternas | Su médico puede prescribir una dosis inicial de 40 mg que puede ser administrada una semana antes de empezar con la pauta habitual. |
Niños y adolescentes desde 2 años que pesen 30 kg o más | 40 mg en semanas alternas | Su médico puede prescribir una dosis inicial de 80 mg que puede ser administrada una semana antes de empezar con la pauta habitual. Se recomienda el uso de Idacio junto con metotrexato. |
Forma y vía de administración
Idacio se administra mediante inyección bajo la piel (vía subcutánea). Para instrucciones de uso, consulte la sección 7 “Instrucciones de uso”.
Si usa más Idacio del que debe
Si accidentalmente se inyecta Idacio con más frecuencia de la que necesita, debe informar de ello a su médico o farmacéutico. Siempre lleve la caja del medicamento consigo, incluso si está vacía.
Si olvidó usar Idacio
Si olvida administrarse una inyección, debe inyectarse la siguiente dosis de Idacio tan pronto como lo recuerde. Después se administrará la siguiente dosis como habitualmente, como si no se hubiese olvidado una dosis.
Si interrumpe el tratamiento con Idacio
La decisión de dejar de usar Idacio debe ser discutida con su médico. Sus síntomas pueden volver tras la interrupción del tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. La mayoría de los efectos adversos son leves a moderados. Sin embargo, algunos pueden ser graves y requerir tratamiento. Los efectos adversos pueden aparecer hasta 4 meses después o más de la última inyección de Idacio.
Busque atención médica urgentemente si nota cualquiera de los siguientes signos de reacción alérgica o insuficiencia cardíaca:
- erupción grave, urticaria;
- hinchazón de la cara, manos, pies;
- dificultad para respirar, tragar;
- falta de aliento al hacer ejercicio o al estar tumbado o hinchazón de pies.
Póngase en contacto con su médico tan pronto como sea posible si nota alguno de los siguientes efectos:
- signos y síntomas de infección tales como fiebre, ganas de vomitar, heridas, problemas dentales, sensación de quemazón al orinar;
- sensación de debilidad o cansancio, tos;
- síntomas de problemas nerviosos como hormigueo, entumecimiento, visión doble o debilidad en brazos o piernas;
- signos de cáncer de piel como una protuberancia o una herida abierta que no se cura;
- signos y síntomas de alteraciones en la sangre como fiebre persistente, cardenales, hemorragias y palidez.
Los siguientes efectos adversos se han observado con adalimumab:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- reacciones en el lugar de inyección (incluyendo dolor, hinchazón, enrojecimiento o picor);
- infecciones del tracto respiratorio inferior (incluyendo resfriado, moqueo, sinusitis, neumonía);
- dolor de cabeza;
- dolor abdominal (de vientre);
- náuseas y vómitos;
- sarpullido;
- dolor en los músculos.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- infecciones graves (incluyendo envenenamiento sanguíneo y gripe);
- infecciones intestinales (incluyendo gastroenteritis);
- infecciones de la piel (incluyendo celulitis y herpes);
- infección de oído;
- infecciones de la boca (incluyendo infección dental y dolor frío);
- infecciones en el sistema reproductor;
- infección del tracto urinario;
- infecciones por hongos;
- infección en las articulaciones;
- tumores benignos;
- cáncer de piel;
- reacciones alérgicas (incluyendo alergia estacional);
- deshidratación;
- cambios de humor (incluyendo depresión);
- ansiedad;
- somnolencia y dificultad para dormir;
- alteraciones sensoriales como hormigueo, escozor o entumecimiento;
- migraña;
- síntomas de compresión de la raíz nerviosa (incluyendo dolor en la parte baja de la espalda y la pierna);
- alteraciones visuales;
- inflamación del ojo;
- inflamación del párpado e hinchazón del ojo;
- vértigo (sensación de que la habitación da vueltas);
- sensación de pulso acelerado;
- alta presión sanguínea;
- rubor;
- hematomas (una hinchazón palpable con sangre coagulada);
- tos;
- asma;
- dificultad para respirar;
- sangrado gastrointestinal;
- dispepsia (indigestión, hinchazón y ardor);
- reflujo ácido;
- síndrome Sicca (que incluye ojos y boca seca);
- picores;
- sarpullido con picor;
- moratones;
- inflamación de la piel (como eczema);
- rotura de uñas de las manos y los pies;
- aumento de la transpiración;
- pérdida de pelo;
- psoriasis de nueva aparición o empeoramiento de la psoriasis existente;
- espasmos musculares;
- sangre en orina;
- problemas renales;
- dolor de pecho;
- edema (acumulación de líquido en el organismo que provoca la hinchazón del tejido afectado);
- fiebre;
- disminución de plaquetas en sangre, lo que incrementa el riesgo de sangrado o moratones;
- problemas de cicatrización.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- infecciones inusuales (que incluyen tuberculosis y otras infecciones) que ocurren cuando la resistencia a la enfermedad disminuye;
- infecciones neurológicas (incluyendo meningitis viral);
- infecciones del ojo;
- infecciones bacterianas;
- diverticulitis (inflamación e infección del intestino grueso);
- cáncer; incluido el cáncer que afecta al sistema linfático (linfoma); y el melanoma (un tipo de cáncer de la piel);
- alteraciones inmunológicas que pueden afectar a los pulmones, piel y ganglios linfáticos (más frecuentemente como una enfermedad llamada sarcoidosis);
- vasculitis (inflamación de los vasos sanguíneos);
- temblor;
- neuropatía (lesión nerviosa);
- derrame cerebral;
- visión doble;
- pérdida de oído, zumbidos;
- sensación de pulso irregular como brincos;
- problemas del corazón que pueden causar dificultad para respirar o hinchazón de tobillos;
- ataque al corazón;
- saco en la pared de una arteria mayor, inflamación y coagulación en una vena, bloqueo de un vaso sanguíneo;
- enfermedades pulmonares que pueden causar dificultad para respirar (incluyendo inflamación);
- embolia pulmonar (bloqueo de una arteria del pulmón);
- derrame pleural (almacenamiento anormal de fluido en el espacio pleural);
- inflamación del páncreas que causa un dolor grave en el abdomen y la espalda;
- dificultad para tragar;
- edema facial (hinchazón);
- inflamación de la vesícula; piedras en la vesícula;
- grasa en el hígado (acumulación de grasa en las células hepáticas);
- sudores nocturnos;
- cicatrices;
- crisis muscular anormal;
- lupus eritematoso sistémico (un trastorno inmunitario que incluye inflamación de la piel, corazón, pulmones, articulaciones y otros órganos);
- interrupciones del sueño;
- impotencia;
- inflamaciones.
Raros(pueden afectar hasta 1 de cada 1000 personas)
- leucemia (cáncer que afecta a la sangre y la médula ósea);
- reacción alérgica grave con shock;
- esclerosis múltiple;
- alteraciones nerviosas (como inflamación del nervio óptico del ojo y síndrome de Guillain-Barré, una enfermedad que puede provocar debilidad muscular, sensaciones anormales, hormigueo en los brazos y la parte superior del cuerpo);
- parada cardiaca;
- fibrosis pulmonar (cicatriz en el pulmón);
- perforación intestinal (agujero en la pared del intestino);
- hepatitis (inflamación del hígado);
- reactivación del virus de la hepatitis B;
- hepatitis autoinmune (inflamación del hígado causada por el propio sistema inmunológico del cuerpo);
- vasculitis cutánea (inflamación de los vasos sanguíneos en la piel);
- síndrome de Stevens-Johnson (reacción potencialmente mortal con síntomas similares a los de la gripe y sarpullido con ampollas);
- edema facial (hinchazón) asociado con reacciones alérgicas;
- eritema multiforme (erupción inflamatoria en la piel);
- síndrome similar al lupus;
- angioedema (inflamación localizada de la piel);
- reacción liquenoide en la piel (sarpullido rojizo-morado con picor)
Frecuencia no conocida(no se puede estimar la frecuencia a partir de los datos disponibles)
- linfoma hepatoesplénico de células T (cáncer sanguíneo raro que a menudo es mortal);
- carcinoma de células de Merkel (un tipo de cáncer de piel);
- fallo hepático;
- empeoramiento de una enfermedad llamada dermatomiositis (visto como erupción cutánea acompañada de debilidad muscular).
Algunos efectos adversos observados en los ensayos clínicos con adalimumab no tienen síntomas y sólo pueden ser identificados mediante un análisis de sangre. Estos incluyen:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- bajo recuento sanguíneo de células blancas;
- bajo recuento sanguíneo de células rojas;
- aumento de lípidos en sangre;
- aumento de enzimas hepáticas.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- alto recuento sanguíneo de células blancas;
- bajo recuento sanguíneo de plaquetas;
- aumento de ácido úrico en sangre;
- valores anormales de sodio en sangre;
- bajo nivel de calcio en sangre;
- bajo nivel de fosfato en sangre;
- azúcar en sangre alto;
- valores altos de lactato deshidrogenasa en sangre;
- presencia de autoanticuerpos en sangre;
- bajo nivel de potasio en sangre.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- valores de bilirrubina elevados (análisis de función hepática).
Raros(pueden afectar hasta 1 de cada 1000 personas)
- recuentos bajos en sangre para células blancas, células rojas y plaquetas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte con su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Idacio
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice la pluma precargada de Idacio después de la fecha de caducidad que aparece en la etiqueta/caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC). No congelar. Conservar la pluma precargada en el embalaje exterior para protegerla de la luz.
Almacenamiento alternativo:
Cuando sea necesario (por ejemplo, cuando esté de viaje), puede almacenar una única pluma precargada de Idacio a temperatura ambiente (hasta 25ºC) durante un periodo máximo de 28 días (asegúrese de protegerlo de la luz). Una vez que se ha sacado de la nevera para almacenar a temperatura ambiente, la pluma precargada, se debe usar en los siguientes 28 días o desechar, incluso si se vuelve a meter más tarde en la nevera.
Debe anotar la fecha en la que retiró la pluma de la nevera, y la fecha después de la cual debe desecharla.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Idacio
El principio activo es adalimumab. Cada pluma precargada contiene 40 mg de adalimumab en 0,8 ml de solución.
Los demás componentes son: Dihidrogenofosfato de sodio dihidrato, fosfato disódico dihidrato, manitol, cloruro de sodio, ácido cítrico, monohidrato, citrato de sodio, polisorbato 80, hidróxido de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Idacio 40 mg solución inyectable en pluma precargada se suministra como 0,8 ml de una solución estéril, incolora y clara de 40 mg de adalimumab.
La pluma precargada de Idacio contiene una jeringa precargada con Idacio. Cada envase contiene 2 ó 6 plumas precargadas con 2 ó 6 toallitas impregnadas en alcohol.
Idacio puede estar disponible en vial, en jeringa precargada y en pluma precargada.
Titular de la autorización de comercialización
Fresenius Kabi Deutschland GmbH
Else-Kröner-Straße 1
61352 Bad Homburg v.d.Höhe
Alemania
Responsable de la fabricación
Fresenius Kabi Austria GmbH
Hafnerstraße 36,
8055 Graz
Austria
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italia
Fecha de la última revisión de este prospecto
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- Instrucciones de uso
Asegúrese de leer, comprender y seguir estas instrucciones de uso antes de inyectar Idacio. Su médico debe mostrarle cómo preparar e inyectar Idacio usando la jeringa precargada antes de utilizar el medicamento por primera vez. Consulte a su médico si tiene alguna duda..
Nota: imágenes sólo con fines ilustrativos
Leer con cuidadado estas instrucciones antes de usar usar su pluma precargada de Idacio.
Información importante
- Sólo utilice Idacio pluma precargada si su profesional sanitario le ha enseñado a usarla correctamente.
- Idacio pluma precargada viene como una pluma precargada lista para un solo uso para dar una dosis completa de adalimumab.
- Inyéctese siempre utilizando la técnica que le enseñó su profesional sanitario.
- Los niños menores de 12 años no pueden inyectarse a ellos mismos y la inyección debe ser realizada por un adulto entrenado.
- Mantenga la pluma precargada Idacio fuera del alcance de los niños.
- No introduzca los dedos en la abertura del resguardo de seguridad.
- No use una pluma precargada Idacio que haya sido congelada o expuesta a la luz directa del sol.
- Hable con su profesional de la salud si tiene alguna pregunta o preocupación.
Información de almacenamiento
- Mantenga la pluma precargada en su caja original para protegerla de la luz.
- Conserve la pluma precargada en la nevera, entre 2°C y 8°C.
- Si es necesario, por ejemplo, cuando se viaja, una única pluma precargada puede almacenarse a temperatura ambiente hasta 14 días.
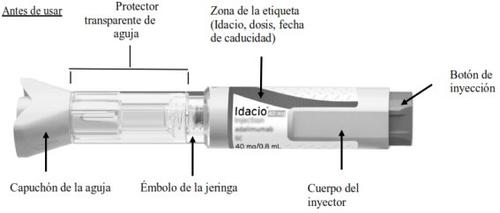
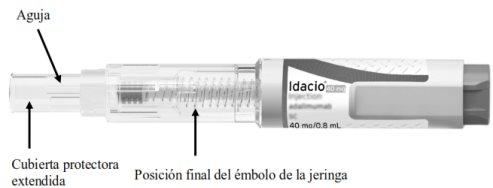
Familiarícese con su pluma precargada Idacio
Después de usar
Paso 1: Prepare su inyección
Cada caja de pluma precargada Idacio viene con dos o seis plumas precargadas.
1.1Prepare una superficie plana y limpia, como una mesa o un mostrador, en una zona bien iluminada.
- Usted también necesitará (Figura A):
? una toallita impregnada en alcohol (incluida en la caja) ? una bola de algodón o gasa, y
? un contenedor para eliminar objetos punzantes.

Figura A
1.3Saque la caja de la nevera (Figura B).

Figura B
1.4Compruebe la fecha de caducidad en el lateral de la caja (Figura C).

Figura C
Advertencia: No usar si la fecha de caducidad ya ha pasado
1.5Saque una pluma precargada del
embalaje original:
- coloque dos dedos en el área de la etiqueta
- tire de la pluma precargada hacia arriba y fuera del embalaje (Figura D).

Figura D
Colóquela sobre una superficie plana y limpia.
1.6Coloque el resto de las plumas en su caja original en la nevera (Figura E).
Figura E
Consulte la información de almacenamiento para saber cómo almacenar su(s) pluma(s) precargada(s) sin usar.
1.7Deje la pluma precargada a temperatura ambiente durante al menos 30 minutos para que el medicamento se atempere. (Figura F).

Figura F
Inyectar medicamentos fríos puede ser doloroso.
Advertencia: Nocaliente la pluma de cualquier otra manera, como en un microondas, agua caliente o luz solar directa.
Advertencia: Noretire la tapa de la aguja hasta que esté listo para inyectarse.
Paso 2: Lávese las manos
2.1Lávese las manos con agua y jabón y séquelas bien. (Figura G).
Advertencia:Los guantes no reemplazarán la necesidad de lavarse las manos.
Figura G |
|
Paso 3: Compruebe la pluma precargada
3.1Compruebe la carcasa transparente de la jeringa para asegurarse de:
- El líquido es transparente, incoloro y libre de partículas (Figura H).
- La jeringa de vidrio no está agrietada ni rota (Figura H).
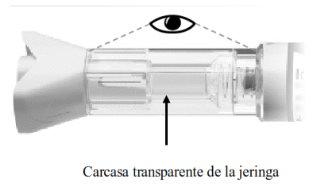
Figura H
Advertencia: Noutilice la pluma precargada si el líquido contiene partículas, si está turbio o si está coloreado, si tiene escamas o si muestra algún signo de daño.
Si es así, deséchelo en un contenedor para eliminar objetos punzantes y póngase en contacto con su profesional sanitario o farmacéutico.
- Compruebe la etiqueta para asegurarse de:
- El nombre en la pluma precargada es Idacio (Figura I).
- La fecha de caducidad en la pluma precargada no ha pasado (Figura I).

Figure I
Advertencia: Nouse la pluma precargada si el nombre en la etiqueta no es Idacio y/o si la fecha de caducidad en la etiqueta ha pasado.
Si es así, deseche la pluma precargada en un contenedor para eliminar objetos punzantes y póngase en contacto con su profesional sanitario o farmacéutico.
Paso 4: Elija el sitio de inyección
- Elija un sitio de inyección (Figura J) en:
- La parte delantera de los muslos.
- Abdomen (inyectar al menos a 5 centímetros de distancia del ombligo).
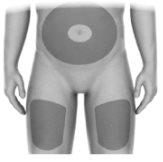
Figura J
4.2Elija un sitio diferente (por lo menos a 2,5 centímetros de distancia del último lugar de inyección) cada vez para reducir el enrojecimiento, la irritación u otros problemas en la piel.
Advertencia: Nose inyecte en un área que esté dolorida (sensible), con cardenales, enrojecida, dura, con cicatrices o donde tenga estrías.
Advertencia:Si usted tiene psoriasis, nose inyecte en ninguna lesión o zonas enrojecidas, engrosadas, levantadas o escamosas.
Paso 5: Limpie el lugar de inyección
5.1Limpie la piel del lugar de la inyección con una toallita con alcohol. (Figura K).
Advertencia: No sople ni toque el lugar de la inyección después de la limpieza.

Figura K
Paso 6: Aplíquese su inyección
- Quite el protector de la aguja
- Sujete la pluma precargada hacia arriba y tire del protector de la aguja hacia fuera. (Figura L)
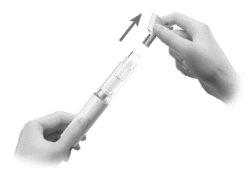
Figura L
Se podrían ver gotas de líquido en la punta de la aguja.
- Deseche el protector de la aguja.
Advertencia: Nogire la tapa.
Advertencia: Novuelva a tapar la pluma precargada.
- Coloque la pluma precargada
- Sostenga la pluma precargada de modo que pueda verse la carcasa transparente de la jeringa.
- Coloque el pulgar encima (sintocar) del botón amarillo de inyección (Figura M).
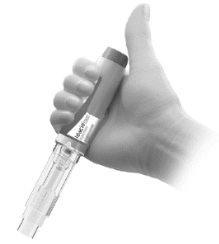
Figura M
- Coloque la pluma precargada contra su piel en un ángulo de 90° (Figura N).

Figura NAntes de la inyección
- Empuje y sostenga la pluma precargada firmemente contra su piel hasta que el protector de seguridad esté presionado por completo. Esto desbloqueará el botón de inyección (Figura O).
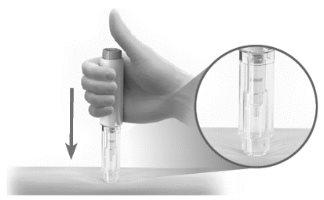
Figura O Antes de la inyección
- Administrar la inyección
- Pulse el botón de inyección (Figura P).
- Escuchará un click, lo que significa que la inyección ha comenzado.
- Continúe sosteniendo firmemente la pluma precargada.
- MIRE el émbolo de la jeringa para asegurarse de que se mueve hasta el fondo. (Figura P).
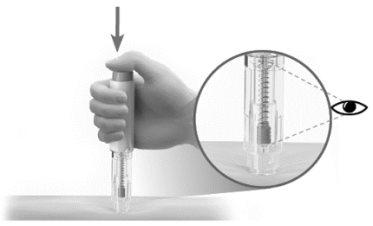
Figura PDespués de la inyección
Advertencia: Nolevante la pluma precargada de la piel hasta que el émbolo haya bajado completamente y se haya inyectado todo el líquido.
- Cuando el émbolo de la jeringa se haya movido hacia el fondo y haya dejado de moverse, continúe sosteniéndolo durante 5 segundos.
- Levante la pluma precargada de la piel (Figura Q).
El protector de seguridad se deslizará hacia abajo y se bloqueará en su lugar para protegerlo de la aguja. (Figura Q).
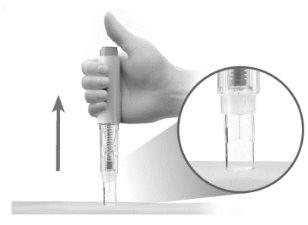
Figura Q
Advertencia: Contacte con su médico o farmacéutico si tiene cualquier problema.
6.4Si hay sangre o líquido en la piel, trate el sitio de la inyección presionando suavemente una bola de algodón o gasa en el sitio (Figura R).

Figura R
Paso 7: Deseche su pluma precargada
7.1Deseche su pluma precargada usada en un contenedor para eliminar objetos punzantes inmediatamente después de su uso (Figura S).

Figura S
Advertencia:Mantenga el contenedor para eliminars objetos punzantes fuera del alcance de los niños.
Advertencia:No tire la pluma precargada a la basura doméstica.
Si no tiene un contenedor para desechar objetos punzantes, puede utilizar un recipiente doméstico que sea:
- Hecho de plástico resistente;
- Pueda cerrarse con una tapa hermética y resistente a los pinchazos; esto evitará que salgan los objetos punzantes.
- Vertical y estable durante el uso,
- Resistente a las fugas y
- Etiquetado correctamente para advertir de la presencia de residuos peligrosos en el interior del recipiente.
7.2Cuando su contenedor para eliminar objetos punzantes esté casi lleno, tendrá que seguir las recomendaciones locales para la correcta eliminación del contenedor.
Norecicle su contenedor para eliminar objetos punzantes usados.
Paso 8: Anote su inyección
8.1Para ayudarle a recordar cuándo y dónde debe realizar su próxima inyección, debe mantener un registro de las fechas y lugares de inyección utilizados para sus inyecciones (Figura T).

Figura T
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IDACIO 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 40 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere receta
Médicos online para IDACIO 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IDACIO 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







