
GELASPAN 40 MG/ML SOLUCION PARA PERFUSION

Cómo usar GELASPAN 40 MG/ML SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Gelaspan 40 mg/ml solución para perfusión
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
|
Contenido del prospecto:
- Qué es Gelaspan y para qué se utiliza
- Qué necesita saber antes de empezar a usar Gelaspan
- Cómo usar Gelaspan
- Posibles efectos adversos
- Conservación de Gelaspan
Contenido del envase e información adicional
1. Qué es Gelaspan y para qué se utiliza
Gelaspan es uno de los llamados sustitutos del volumen plasmático. Esto significa que sustituye el líquido perdido de los vasos sanguíneos.
Gelaspan se utiliza para:
- Sustituir la sangre y los líquidos corporales que se han perdido después de, por ejemplo, una operación, un accidente o una quemadura. Se puede combinar con transfusiones de sangre en caso necesario.
- Prevenir la tensión arterial baja (hipotensión) que se puede producir cuando se recibe anestesia raquídea o epidural o debida a una pérdida grave e inminente de sangre en un marco quirúrgico.
Llenar el volumen de sangre circulante durante el uso de, por ejemplo, un sistema de circulación extracorpórea con otros líquidos para perfusión.
2. Qué necesita saber antes de empezar a usar Gelaspan
No use Gelaspan
- si es alérgico a la gelatina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si es alérgico a un alérgeno llamado “galactosa-α-1,3-galactosa (alfa-Gal)” o a la carne roja (carne de mamífero) y los despojos
- si su volumen de sangre circulante es demasiado alto
- si tiene demasiada agua en su cuerpo
- si tiene ciertos tipos de insuficiencia cardiaca (insuficiencia cardiaca congestiva aguda)
- si tiene un nivel de potasio en sangre anormalmente alto.
Advertencias y precauciones
Consulte a su médico o farmacéutico o enfermero antes de empezar a usar Gelaspan.
Informe a su médico
- si padece una enfermedad alérgica como el asma, ya que puede estar en mayor riesgo de experimentar una reacción alérgica
- en estos casos, no se le debe administrar Gelaspan debido a posibles reacciones cruzadas:
- si sabe que es alérgico a la carne roja (carne de mamífero) o a los despojos
- si ha dado positivo a anticuerpos (IgE) contra el alérgeno alfa-Gal
Su médico tendrá especial cuidado con su situación si sufre de
- problemas de corazón
- presión arterial alta
- agua en sus pulmones
- problemas renales graves.
Administrar grandes cantidades de líquidos por un goteo intravenoso puede empeorar su estado.
Su médico también actuará con precaución
- si tiene un aumento grave de sodio o de cloruro en la sangre
- si retiene agua y sal, lo cual puede estar asociado a inflamación de los tejidos.
- si tiene demasiado potasio en la sangre o si está tomando o recibiendo medicamentos que causan retención de potasio
- si la coagulación de su sangre está gravemente afectada
- si es usted una persona de edad avanzada
Mientras esté recibiendo Gelaspan, se vigilará la composición de su sangre. En caso necesario, es posible que su médico le administre otros medicamentos tales como sales y líquidos.
Niños:
Existe poca experiencia sobre el uso de Gelaspan en niños. El médico únicamente administrará este medicamento a los niños cuando crea que es absolutamente necesario.
Resultados de pruebas de laboratorio
Es posible que su médico le tome muestras de sangre o de orina antes de administrarle Gelaspan. Esto se debe a que algunos resultados de pruebas de laboratorio pueden verse afectados después de haber recibido este medicamento y, por tanto, no son fiables.
Otros medicamentos y Gelaspan
Informe a su médico o farmacéutico si está tomando o utilizando o ha tomado o utilizado recientemente cualquier otro medicamento, incluso los adquiridos sin receta.
En particular, su médico debe conocer si usted está tomando o recibiendo medicamentos que le hagan retener sodio (por ejemplo, espironolactona, triamtereno, amilorida; inhibidores de la ECA como el captopril o el enalapril, corticosteroides como la cortisona o antiinflamatorios no esteroideos como el diclofenaco), La administración concomitante con este medicamento podría causar hinchazón de los brazos, las manos, las piernas y los pies (edema). Además, informe a su médico si está tomando medicamentos que le hagan perder potasio tales como medicamentos que aumentan la pérdida de agua.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de tomar cualquier otro medicamento.
Embarazo
Si está embarazada, informe a su médico. Debido a la posibilidad de reacciones alérgicas, debe evitarse el uso de este medicamento durante el embarazo. Sin embargo, su médico puede administrarle este medicamento en situaciones de urgencia.
Lactancia
Si está en periodo de lactancia, informe a su médico. Se dispone de información limitada sobre la excreción de este medicamento en la leche materna. Su médico decidirá si es necesario interrumpir la lactancia o interrumpir el tratamiento con este medicamento tras considerar el beneficio de la lactancia para el niño y el beneficio del tratamiento para usted.
Fertilidad
No se dispone de datos sobre el efecto de este medicamento en la fertilidad humana o animal. Sin embargo, debido a la naturaleza de sus componentes, se considera improbable que afecte a la fertilidad.
Conducción y uso de máquinas
El medicamento no influye en la capacidad para conducir o usar máquinas..
3. Cómo usar Gelaspan
Su médico solo le administrará Gelaspan si considera que otros productos denominados cristaloides no son suficientes por sí solos.
Su médico ajustará con cuidado la dosis de Gelaspan para prevenir la sobrecarga de líquidos. Esto se hará especialmente si tiene problemas en los pulmones, el corazón o la circulación.
Posología
Gelaspan se administra por vía intravenosa, es decir, gota a gota.
Adultos
Cuánto volumen le administren y durante cuánto tiempo dependerá de la cantidad de sangre o líquido que haya perdido y de su estado.
El médico le realizará pruebas (por ejemplo, para la tensión arterial y la sangre) durante el tratamiento y le ajustará la dosis de Gelaspan de acuerdo con sus necesidades. En caso necesario, puede recibir también sangre o concentrados de hematíes.
Uso en niños
Existe poca experiencia en el uso de este medicamento en niños. El médico solo administrará este medicamento si lo considera esencial para la recuperación del niño. En estos casos, se tendrá en cuenta el estado clínico y se vigilará el tratamiento con especial cuidado.
Si ha recibido más Gelaspan del que debe
Una sobredosis de Gelaspan puede causar un volumen de sangre demasiado alto (hipervolemia) y sobrecarga de líquido que pueden afectar al corazón y a la función pulmonar.
Si se produjo una sobredosis, su médico le administrará el tratamiento necesario.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Gelaspan puede producir efectos adversos, aunque no todas las personas los sufran.
Todos los sustitutos del plasma conllevan un ligero riesgo de reacciones alérgicas, generalmente leves o moderadas, pero en muy pocas ocasiones también pueden ser graves. Se presupone que estas reacciones son más frecuentes en pacientes con trastornos alérgicos conocidos, como el asma. Por ese motivo, usted será vigilado estrechamente por un profesional sanitario, especialmente al comienzo de la perfusión.
Los siguientes efectos adversos pueden ser graves. Si se produce alguno de los siguientes efectos adversos, consulte a un médico inmediatamente:
Raros (pueden afectar a hasta 1 de cada 1.000 personas):
Reacciones alérgicas (anafilácticas/anafilactoides) que incluyen, por ejemplo, dificultad al respirar, sibilancias, náuseas, vómitos, mareos, sudoración, opresión en el pecho o en la garganta, dolor de estómago, hinchazón del cuello y la cara.
Si se produce una reacción alérgica, se detendrá inmediatamente la perfusión y se le administrará el tratamiento necesario (ver también sección 2 "Qué necesita saber antes de empezar a usar Gelaspan", especialmente para las alergias en las que están implicados los alérgenos llamados galactosa-α-1,3-galactosa (alfa-Gal), las carnes rojas y los despojos).
Otros efectos adversos incluyen:
Muy frecuentes (pueden afectar a hasta 1 de cada 10 personas):
- disminución de los glóbulos rojos y de las proteínas de la sangre
Frecuentes (pueden afectar a hasta 1 de cada 100 personas):
- es posible que su sangre no coagule tan bien como antes y que note que sangra con más facilidad
Muy raras (pueden afectar a hasta 1 de cada 10.000 personas):
- aceleración del latido cardiaco
- presión arterial baja
- fiebre, escalofríos.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- náuseas, vómitos, dolor de estómago
- disminución del oxígeno en la sangre que puede hacer que se sienta mareado
Otros efectos adversos en niños
No existen datos relativos a una diferencia en los efectos adversos en niños.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano. www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Gelaspan
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice Gelaspan después de la fecha de caducidad que aparece en la etiqueta y en la caja. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25 ºC. No congelar.
No utilice Gelaspan si observa:
- turbidez o decoloración de la solución
- fuga del envase.
Se debe desechar el Gelaspan previamente abierto o parcialmente utilizado. No se deben volver a conectar los frascos o bolsas parcialmente utilizados.
6. Contenido del envase e información adicional
Composición de Gelaspan
Principios activos:
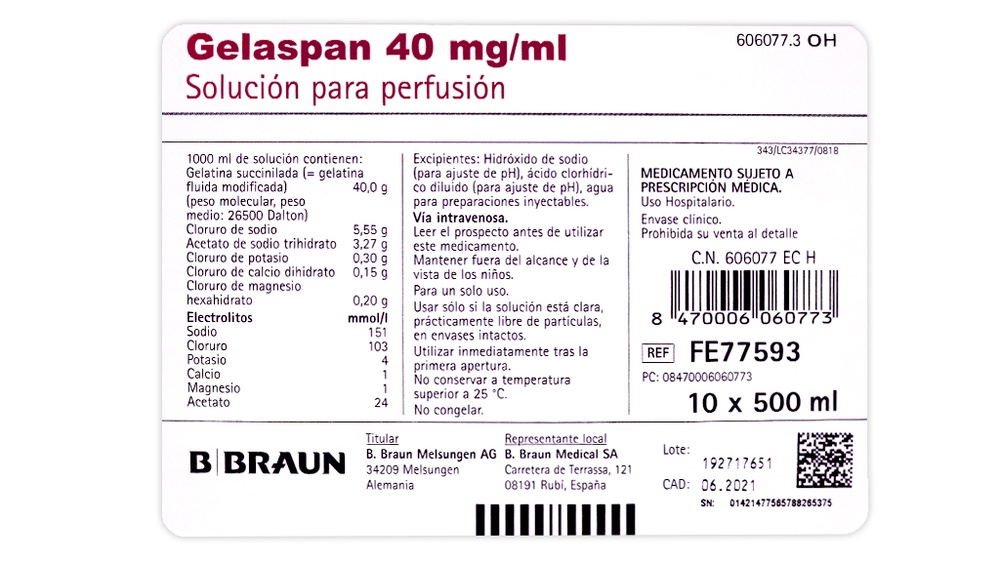
1.000 ml de solución contienen:
Gelatina succinilada (fluida modificada) 40,0 g
Cloruro de sodio 5,55 g
Acetato de sodio trihidrato 3,27 g
Cloruro de potasio 0,30 g
Cloruro de calcio dihidrato 0,15 g
Cloruro de magnesio hexahidrato 0,20 g
Concentración electrolítica
Sodio 151 mmol/l
Cloruro 103 mmol/l
Potasio 4 mmol/l
Calcio 1 mmol/l
Magnesio 1 mmol/l
Acetato 24 mmol/l
Los demás componentes son:
Agua para preparaciones inyectables, ácido clorhídrico diluido (para ajuste del pH) e hidróxido de sodio (para ajuste del pH).
Aspecto del producto y contenido del envase
Gelaspan es una solución para perfusión administrada por goteo intravenoso (goteo en una vena).
Se trata de una solución estéril, transparente e incolora o ligeramente amarillenta.
Gelaspan se presenta en:
- Frascos de polietileno de baja densidad “Ecoflac plus”, que contienen 500 ml disponibles en envases de 10 x 500 ml
- Bolsas de plástico “Ecobag” (sin PVC), selladas con tapones de goma, que contienen 500 ml disponibles en envases de 20 x 500 ml.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización, responsable de la fabricación y representante local
Titular y responsable de la fabricación
- Braun Melsungen AG
Carl-Braun-Straße 1
34212 Melsungen, Alemania
Dirección postal
34209 Melsungen, Alemania
Teléfono: +49/5661/71-0
Fax: +49/5661/71-4567
Fabricanteresponsablede la liberación del lote en UK
- Braun Medical Limited
Brookdale Road
Thorncliffe Park Estate
Chapeltown
Sheffield
S35 2PW
United Kingdom
Fabricante responsable de la liberación de lotes en ES y PT
- Braun Medical S.A.
Carretera de Terrassa, 121
08191 Rubí (Barcelona)
España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización
- Braun Medical, SA
Ctra. Terrassa, 121
08191 Rubí (España)
Este medicamento se ha autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Nombre del estado miembro | Nombre del medicamento |
Austria | Gelofusin Iso 40 mg/ml Infusionslösung |
Bélgica | Isogelo oplossing voor infusie, solution pour perfusion, Infusionslösung |
Bulgaria | Gelofusine Balance 4% solution for Infusion |
República Checa | Gelaspan 4% |
Dinamarca | Gelaspan |
Estonia | Gelaspan infusioonilahus 4% |
Francia | Gelaspan, solution pour perfusion |
Alemania | Gelafundin ISO 40 mg/ml Infusionslösung |
Grecia | Gelaspan solution for Infusion 4% |
Hungría | Gelaspan 4% oldatos infúzió |
Irlanda | Gelaspan Solution for Infusion |
Italia | Gelaspan |
Letonia | Gelaspan 4% Solution for Infusion |
Lituania | Gelaspan 4% infuzinis tirpalas |
Luxemburgo | Gelafundin ISO 40 mg/ml Infusionslösung |
Malta | Gelaspan 4% Solution for Infusion |
Países Bajos | Gelaspan, oplossing voor infusie |
Noruega | Gelaspan |
Polonia | Gelaspan |
Portugal | Gelaspan |
Rumanía | Gelaspan 40 mg/ml solutie perfuzabila |
Eslovaquia | Gelaspan 4% |
Eslovenia | Gelaspan 40 mg/ml raztopina za infundiranje |
España | Gelaspan 40 mg/ml solución para perfusión |
Suecia | Gelaspan |
Reino Unido (Irlanda del Norte) | Gelaspan solution for infusion |
Fecha de la última revisión de este prospecto:abril 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios http://www.aemps.gob.es/
Esta información está destinada únicamente a profesionales del sector sanitario:
Precauciones de empleo
Gelaspan no se debe perfundir a través de la misma vía de perfusión con sangre, componentes sanguíneos o hemoderivados (concentrados de hematíes, plasma y fracciones plasmáticas).
Durante la compensación de las pérdidas graves de sangre por perfusiones de grandes cantidades de Gelaspan se deben vigilar el hematocrito y los electrolitos. El hematocrito no debe disminuir por debajo del 25%. En pacientes de edad avanzada o gravemente enfermos, no debe ser inferior al 30%.
Asimismo, se debe observar el efecto de dilución de los factores de coagulación en esas situaciones, especialmente en pacientes con trastornos existentes de la hemostasia.
Debido a que el producto no sustituye las pérdidas de proteínas plasmáticas, es recomendable controlar las concentraciones de proteínas plasmáticas.
En situaciones agudas graves, Gelaspan se puede perfundir rápidamente mediante perfusión por presión (se pueden administrar 500 ml en 5‑10 minutos) hasta que se alivien los signos de hipovolemia.
Antes de una perfusión rápida, Gelaspan se puede calentar a no más de 37 °C.
En el caso de la perfusión por presión, que puede ser necesaria en situaciones de emergencia vital, se debe eliminar todo el aire del envase y del equipo de perfusión antes administrar la solución para evitar el riesgo de embolia gaseosa que, de lo contrario, podría asociarse a la perfusión.
Influencia en las pruebas de laboratorio
Las pruebas sanguíneas de laboratorio (grupo sanguíneo y antígenos irregulares) son posibles después de las perfusiones de Gelaspan. No obstante, se recomienda tomar muestras de sangre antes de la perfusión de Gelaspan con el fin de evitar una interpretación dudosa de los resultados.
Gelaspan puede influir en las siguientes pruebas químico-clínicas, dando lugar a valores falsamente elevados:
- velocidad de sedimentación eritrocitaria
- peso específico de la orina
- ensayos inespecíficos de proteínas, por ejemplo, el método de Biuret.
Incompatibilidades
En ausencia de estudios de compatibilidad, este medicamento no se debe mezclar con otros medicamentos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GELASPAN 40 MG/ML SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 3 g; 0,5382 g; 0,0305 g; 0,0373 g; 0,3360 gPrincipio activo: gelatin agentsFabricante: Fresenius Kabi España, S.A.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 200 g/lPrincipio activo: AlbuminaFabricante: Biotest Pharma GmbhRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 200 g/lPrincipio activo: AlbuminaFabricante: Csl Behring GmbhRequiere receta
Médicos online para GELASPAN 40 MG/ML SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GELASPAN 40 MG/ML SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















