GALAFOLD 123 MG CAPSULAS DURAS

Cómo usar GALAFOLD 123 MG CAPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Galafold 123 mg cápsulas duras
Migalastat
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos signos que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Galafold y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Galafold
- Cómo tomar Galafold
- Posibles efectos adversos
- Conservación de Galafold
- Contenido del envase e información adicional
1. Qué es Galafold y para qué se utiliza
Galafold contiene el principio activo migalastat.
Este medicamento se utiliza para el tratamiento a largo plazo de la enfermedad de Fabry en adultos y adolescentes de 12 años de edad y mayores portadores de determinadas mutaciones genéticas (cambios en el material genético).
La enfermedad de Fabry se debe a un déficit de una enzima denominada alfa-galactosidasa A (α-Gal A). En función del tipo de mutación (cambio en el material genético) del gen que produce la α-Gal A, la enzima puede no funcionar correctamente o estar totalmente ausente. Este déficit de la enzima provoca acumulaciones anómalas de una sustancia grasa conocida como globotriaosilceramida (GL-3) en riñones, corazón y otros órganos, causando los síntomas de la enfermedad de Fabry.
Este medicamento actúa estabilizando la enzima que el cuerpo produce naturalmente, de modo que pueda funcionar mejor para reducir la cantidad de GL-3 acumulada en células y tejidos.
2. Qué necesita saber antes de empezar a tomar Galafold
No tome Galafold:
- si es alérgico a migalastat o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Las cápsulas de 123 mg de migalastat no están indicadas para niños (≥12 años) de menos de
45 kg de peso corporal.
Informe a su médico antes de tomar Galafold si está utilizando terapia de sustitución enzimática.
No debe tomar Galafold si también está utilizando terapia de sustitución enzimática.
Mientras esté tomando Galafold, su médico debe valorar su estado y si su medicamento está funcionando cada 6 meses. Si su estado empeora, su médico debe realizarle pruebas adicionales o interrumpir el tratamiento con Galafold.
Consulte a su médico antes de tomar Galafold si su funcionamiento renal está gravemente disminuido, ya que no se recomienda utilizar Galafold en pacientes con insuficiencia renal grave (con una TFG por debajo de 30 ml/min/1,73 m2).
Niños
Niños <12 años
Este medicamento no ha sido estudiado en niños menores de 12 años. Por consiguiente, no se ha establecido la seguridad y la eficacia en esta franja de edad.
Otros medicamentos y Galafold
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o podría tomar cualquier otro medicamento. Esto incluye medicamentos adquiridos sin receta, como suplementos u medicamentos a base de hierbas.
Informe a su médico especialmente si toma medicamentos o suplementos que contienen cafeína, ya que estos medicamentos pueden afectar a la actividad de Galafold si se toman en el periodo de ayuno.
Sea consciente de los medicamentos que toma. Lleve una lista de ellos y enséñesela al médico y al farmacéutico cada vez que obtenga un medicamento nuevo.
Embarazo, lactancia y fertilidad
Embarazo
Existen datos muy limitados respecto a la utilización de este medicamento en mujeres embarazadas. No se recomienda el uso de Galafold durante el embarazo. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Las mujeres que puedan quedarse embarazadas deben utilizar un método anticonceptivo eficaz mientras usen Galafold.
Lactancia
No tome este medicamento si está dando el pecho a su hijo hasta que haya consultado con su médico, farmacéutico o enfermero. No se sabe si este medicamento se excreta en la leche materna. Su médico decidirá si debe interrumpir la lactancia o suspender el tratamiento temporalmente, considerando el beneficio de la lactancia para el bebé y el beneficio de Galafold para la madre.
Fertilidad en varones
No se sabe si este medicamento afecta a la fertilidad masculina. No se han estudiado los efectos de Galafold sobre la fertilidad en el ser humano.
Fertilidad en mujeres
No se sabe si este medicamento afecta a la fertilidad femenina.
Si tiene previsto quedarse embarazada, consulte con su médico, farmacéutico o enfermero.
Conducción y uso de máquinas
No se espera que este medicamento afecte a su capacidad para conducir y utilizar máquinas.
3. Cómo tomar Galafold
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte a su médico, farmacéutico o enfermero.
La dosis recomendada es una cápsula cada dos días a la misma hora del día. No tome Galafold durante dos días consecutivos.
No ingiera ningún alimento ni cafeína como mínimo 2 horas antes y 2 horas después de tomar el medicamento. Este ayuno mínimo de 4 horas alrededor de la toma del medicamento es necesario para permitir que se absorba el medicamento por completo.
Durante el periodo de ayuno de 4 horas, se pueden consumir agua (natural, saborizada, azucarada), sumos de frutas sin pulpa y bebidas con gas sin cafeína.
Tragar las cápsulas enteras. No partir, triturar ni masticar las cápsulas.
Figura A
| Paso 1: Retire el precinto adhesivo que mantiene la solapa cerrada. Levante la solapa para abrir el cartón de Galafold (ver figura A). |
Figura B: cartón abierto
| Paso 2: Presione la pestaña morada con el pulgar en el lado izquierdodel cartón sin soltarla (ver figura B) y siga con el paso 3. |
Figura C
| Paso 3: Ahora AGARRE la pestaña por el lado derecho, donde dice “TIRAR DE AQUÍ”, y saque el blíster doblado (ver figura C). |
Figura D: cara frontal del blíster
| Paso 4: Desdoble el blíster (ver figura D). |
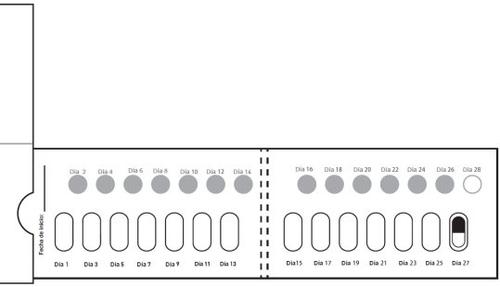
Cómo tomar las cápsulas de Galafold: Un blíster de Galafold = 14 cápsulas duras = 28 días de tratamiento con Galafold y 14 círculos blancos. Los círculos blancos sirven para recordarle que debe tomar Galafold cada dosdías. La flecha indica al paciente que debe empezar las 2 semanas de tratamiento siguientes. Figura E: cara frontal del blíster
| |
Figura F: cara frontal del blíster
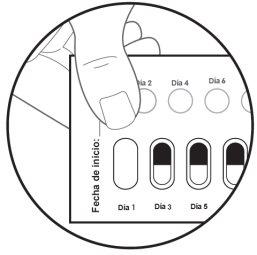
| Paso 5: El primer día que comience con este medicamento, marque la fecha en el blíster nuevo. (ver figura F). |
Figura G: cara posterior del blíster
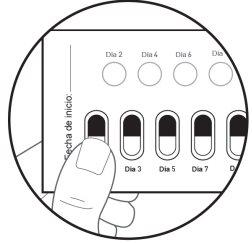
| Paso 6: DÉ LA VUELTA al blíster para ver la cara posterior. LOCALICE la cápsula que va a extraer. DOBLE el blíster como se indica aquí (ver figura G). Nota: Al doblar el blíster sobresale el óvalo perforado. |
| Paso 7: RETIRE el óvalo perforado figura H). Nota: Al retirar el óvalo es posible que la lámina de soporte blanca aún permanezca. Esto es correcto. |
Figura I: cara frontal del blíster
| Paso 8: DÉ LA VUELTA al blíster para ver la cara frontal. EMPUJE la cápsula para sacarla (ver figura I). |
Figura J: cara frontal del blíster
| Paso 9: Al día siguiente, pase al círculo blanco de la fila de arriba señalado como Día 2. Presione el círculo blanco para perforarlo (ver figura J). Nota: Perforar este círculo blanco le ayudará a recordar qué días no debe tomar el medicamento. Tome 1 cápsula de Galafold una vez cada dosdías. Cierre y guarde la caja después de cada uso. |
Después del Día 2, pase al Día 3 del blíster. Alterne los días que toma la cápsula y perfora los círculos blancos, hasta el día 28, incluido este último. Figura K: cara frontal del blíster desdoblado
|
Si toma más Galafold del que debe
Si toma más cápsulas de las que debe, deje de tomar el medicamento y consulte con su médico. Es posible que experimente dolor de cabeza y vértigos.
Si olvidó tomar Galafold
Si olvidó tomar la cápsula a la hora habitual y se acordó posteriormente se puede tomar la cápsula solamente si está dentro del intervalo de las 12 horas siguientes a la hora habitual a la que se toma la dosis. Si han transcurrido más de 12 horas, deberá volver a tomar Galafold el día correspondiente a la siguiente dosis y a la hora habitual, de acuerdo con la pauta posológica de días alternos. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Galafold
No debe interrumpir el tratamiento sin hablar antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- Dolor de cabeza
Frecuentes: pueden afectar hasta 1 de cada 10 personas
|
|
|
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Galafold
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna temperatura especial de conservación. Conservar en el embalaje original para protegerlo de la humedad.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Galafold
- El principio activo es migalastat. Cada cápsula contiene clorhidrato de migalastat equivalente a 123 mg de migalastat
- Los demás componentes son:
Contenido de la cápsula: almidón pregelatinizado (maíz) y estearato de magnesio
Cubierta de la cápsula: gelatina, dióxido de titanio (E171) e carmín de índigo (E132)
Tinta de impresión: goma laca, óxido de hierro negro e hidróxido de potasio
Aspecto de Galafold y contenido del envase
Cápsulas duras azules y blancas opacas marcadas con «A1001» en tinta negra, cápsula dura de tamaño 2 (6,4 x 18,0 mm) que contiene un polvo de blanco a marrón claro.
Galafold está disponible en un blíster de 14 cápsulas.
Titular de la autorización de comercialización
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Irlanda
Tel: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
e-mail: [email protected]
Fabricante
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate
Dundalk, Co. Louth
A91 P9KD
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización (si no puede contactar con su representante de Amicus por teléfono, póngase en contacto a través de la dirección de correo electrónico que figura a continuación):
België/Belgique/Belgien Amicus Therapeutics Europe Limited Tél/Tel: (+32) 0800 89172 e-mail: [email protected] | Lietuva Amicus Therapeutics Europe Limited Tel.: (+370) 8800 33167 El. paštas: [email protected] |
???????? Amicus Therapeutics Europe Limited Te?.: (+359) 00800 111 3214 ?????: [email protected] | Luxembourg/Luxemburg Amicus Therapeutics Europe Limited Tél/Tel: (+352) 800 27003 e-mail: [email protected] |
Ceská republika Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 e-mail: [email protected] | Magyarország Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 e-mail: [email protected] |
Danmark Amicus Therapeutics Europe Limited Tlf.: (+45) 80 253 262 e-mail: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 e-mail: [email protected] |
Deutschland Amicus Therapeutics GmbH Tel.: (+ 49) 0800 000 2038 E-Mail: [email protected] | Nederland Amicus Therapeutics BV Tel: (+ 31) 0800 022 8399 e-mail: [email protected] |
Eesti Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-post: [email protected] | Norge Amicus Therapeutics Europe Limited Tlf: (+47) 800 13837 e-post: [email protected] |
Ελλáδα Amicus Therapeutics Europe Limited Τηλ.: (+30) 00800 126 169 e-mail: [email protected] | Österreich Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 E-Mail: [email protected] |
España Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 e-mail: [email protected] | Polska Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 e-mail: [email protected] |
France Amicus Therapeutics SAS Tél: (+33) 0 800 906 788 e-mail: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 e-mail: [email protected] |
Hrvatska Amicus Therapeutics Europe Limited Tel: (+385) 0800 222 452 e-pošta: [email protected] | România Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 e-mail: [email protected] |
Ireland Amicus Therapeutics Europe Limited Tel: (+354) 1800 936 230 e-mail: [email protected] | Slovenija Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-pošta: [email protected] |
Ísland Amicus Therapeutics Europe Limited Sími: (+354) 800 7634 Netfang: [email protected] | Slovenská republika Amicus Therapeutics Europe Limited Tel.: (+421) 0800 002 437 e-mail: [email protected] |
Italia Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 e-mail: [email protected] | Suomi/Finland Amicus Therapeutics Europe Limited Puh/Tel: (+358) 0800 917 780 sähköposti/e-mail: [email protected] |
Κúπρος Amicus Therapeutics Europe Limited Τηλ.: (+357) 800 97595 e-mail: [email protected] | Sverige Amicus Therapeutics Europe Limited Tfn: (+46) 020 795 493 e-post: [email protected] |
Latvija Amicus Therapeutics Europe Limited Tel.: (+371) 800 05391 e-pasts: [email protected] | United Kingdom (Northern Ireland) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 e-mail: [email protected] |
Fecha de la última revisión de este prospecto
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GALAFOLD 123 MG CAPSULAS DURASForma farmacéutica: COMPRIMIDO, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: CAPSULA, 84 MG de eliglustat (como tartrato)Principio activo: EliglustatFabricante: Sanofi B.V.Requiere receta
Médicos online para GALAFOLD 123 MG CAPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GALAFOLD 123 MG CAPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes




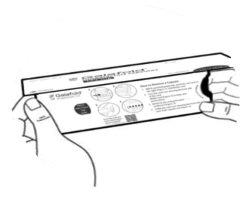
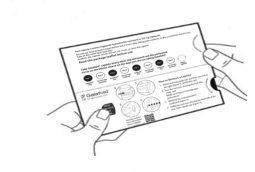

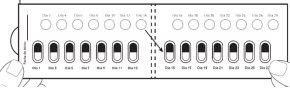
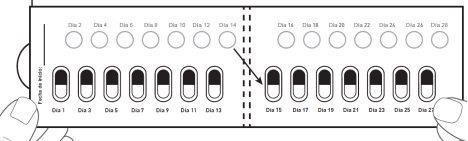
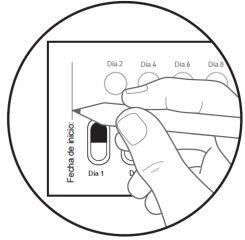
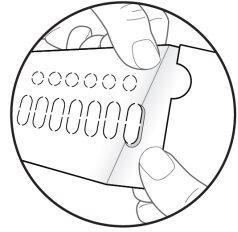
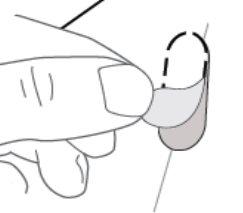 Figura H: cara posterior del blíster
Figura H: cara posterior del blíster (ver
(ver