EVENITY 105 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar EVENITY 105 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
EVENITY 105 mg solución inyectable en pluma precargada
romosozumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Se le proporcionará una tarjeta de alerta del paciente que contiene información de seguridad importante que necesita conocer antes y durante su tratamiento con EVENITY.
Contenido del prospecto
- Qué es EVENITY y para qué se utiliza
- Qué necesita saber antes de empezar a usar EVENITY
- Cómo usar EVENITY
- Posibles efectos adversos
- Conservación de EVENITY
- Contenido del envase e información adicional
1. Qué es EVENITY y para qué se utiliza
Qué es EVENITY
Evenity contiene el principio activo romosozumab, un medicamento que contribuye a fortalecer los huesos y a reducir su riesgo de fractura.
Para qué se utiliza EVENITY
EVENITY se utiliza para el tratamiento de la osteoporosis grave en mujeres después de la menopausia que tengan un riesgo elevado de sufrir una fractura.
La osteoporosis es una enfermedad que hace que los huesos se vuelvan frágiles y quebradizos. Muchas pacientes con osteoporosis no muestran ningún síntoma, pero pueden presentar un mayor riesgo de fracturas.
Cómo funciona EVENITY
EVENITY es un anticuerpo monoclonal. Un anticuerpo monoclonal es un tipo de proteína que se ha diseñado para reconocer y fijarse a proteínas específicas en el cuerpo. EVENITY se une a una proteína denominada esclerostina. Al unirse a ella y bloquear su actividad, EVENITY:
- contribuye a formar hueso nuevo y
- frena la pérdida del hueso existente.
Esto hace que los huesos sean más fuertes y reduce su riesgo de fractura.
2. Qué necesita saber antes de empezar a usar EVENITY
No use EVENITY
- si es alérgica a romosozumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si presenta niveles bajos de calcio en la sangre (hipocalcemia). Su médico podrá decirle si sus niveles son demasiado bajos;
- si tiene antecedentes de infarto de miocardio o accidente cerebrovascular.
No use EVENITY si se encuentra en alguna de las circunstancias anteriores. Si no está segura, consulte con su médico o farmacéutico antes de usar EVENITY.
Advertencias y precauciones
Consulte a su médico o farmacéutico y comente sus antecedentes médicos antes de empezar a usar EVENITY.
Infarto de miocardio y accidente cerebrovascular
En las personas que reciben EVENITY se han notificado infarto de miocardio y accidente cerebrovascular.
Busque atención médica de inmediatosi presenta:
- dolor en el pecho, dificultad para respirar;
- dolor de cabeza, entumecimiento o debilidad en rostro, brazos o piernas, dificultad para hablar, cambios en la visión, pérdida de equilibrio.
Su médico evaluará detenidamente el riesgo de problemas cardiovasculares antes de permitirle comenzar el tratamiento con EVENITY. Informe a su médico si sabe que tiene un riesgo elevado de sufrir problemas cardiovasculares tales como enfermedad cardiovascular establecida, presión arterial alta, niveles altos de grasa en la sangre, diabetes, tabaquismo o problemas renales.
Niveles bajos de calcio en la sangre
EVENITY puede provocar niveles bajos de calcio en la sangre.
Informe a su médicosi observa:
- espasmos, tirones o calambres musculares;
- entumecimiento u hormigueo en los dedos de las manos o de los pies, o alrededor de la boca.
Su médico puede recetarle calcio y vitamina D para ayudarle a prevenir los niveles bajos de calcio en la sangre antes de que comience el tratamiento y mientras toma EVENITY. Tome calcio y vitamina D según se lo indique su médico. Indique a su médico si presenta o ha presentado en alguna ocasión problemas renales graves, insuficiencia renal o ha necesitado diálisis, dado que esto puede aumentar su riesgo de presentar una concentración baja de calcio en la sangre si no toma suplementos de calcio.
Reacciones alérgicas graves
Las personas que utilizan EVENITY pueden sufrir reacciones alérgicas graves.
Busque atención médica de inmediatosi presenta:
- hinchazón del rostro, la boca, la garganta, las manos, los pies, los tobillos, la parte inferior de las piernas (angioedema), o ronchas;
- erupción cutánea aguda que muestra múltiples puntos rojos/rosas redondos con una ampolla o una costra en el centro (eritema multiforme);
- dificultad para tragar o respirar.
Problemas en la boca, los dientes o la mandíbula
En las pacientes que reciben EVENITY se ha notificado en raras ocasiones (puede afectar a hasta 1 de cada 1000 personas) un efecto adverso denominado osteonecrosis de la mandíbula (ONM, daños óseos en la mandíbula). La ONM puede también producirse después de detener el tratamiento. Es importante intentar prevenir la aparición de la ONM, dado que puede ser un trastorno doloroso que puede resultar difícil de tratar. Para reducir el riesgo de aparición de ONM, deberá tomar algunas precauciones.
Antes de recibir EVENITY, avise a su médico o enfermero si:
- presenta algún problema con la boca o los dientes, como mala salud dental, gingivitis o tiene prevista una extracción;
- no recibe atención dental habitual o no se ha sometido a una revisión dental durante mucho tiempo;
- es fumadora (dado que esto puede aumentar el riesgo de problemas dentales);
- ha recibido con anterioridad tratamiento con bifosfonatos (que se usan para tratar o prevenir los trastornos óseos como la osteoporosis);
- está tomando medicamentos denominados corticoesteroides (como prednisolona o dexametasona);
- tiene cáncer.
Es posible que su médico le pida que se someta a una exploración dental antes de comenzar el tratamiento con EVENITY.
Durante el tratamiento, deberá mantener una buena higiene oral y someterse a revisiones dentales periódicas. Si lleva dentadura postiza, deberá asegurarse de que esta encaja correctamente. Si está recibiendo tratamiento dental o va a someterse a una intervención dental (como una extracción dental), informe a su médico de su tratamiento dental y avise a su dentista de que está recibiendo tratamiento con EVENITY.
Póngase inmediatamente en contacto con su médico y su dentista si tiene algún problema en la boca o los dientes, tales como:
- movimiento de los dientes;
- dolor o hinchazón;
- úlceras orales que no cicatrizan;
- supuración.
Fracturas atípicas del fémur
En las personas que han usado EVENITY, en raras ocasiones aparecieron fracturas atípicas del fémur causadas por un pequeño o ningún traumatismo. Estos tipos de fracturas a menudo se vieron precedidas de señales de advertencia como dolor en el fémur o en la ingle durante varias semanas antes de que se produjese la fractura. Se desconoce si EVENITY provocó estas fracturas inusuales. Avise a su médico o farmacéutico si presenta algún dolor nuevo o inusual en su cadera, la ingle o el muslo.
Niños y adolescentes
No se ha estudiado el uso de romosozumab en niños y adolescentes y no está aprobado para usarse en pacientes pediátricos (edad < 18 años).
Otros medicamentos y EVENITY
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o es posible que vaya a tomar otros medicamentos.
Embarazo y lactancia
Se espera que EVENITY se utilice solamente para tratar a mujeres posmenopáusicas.
No debe utilizarse EVENITY en mujeres en edad fértil, ni durante el embarazo o la lactancia. Se desconoce si EVENITY puede hacer daño al feto o al lactante. Póngase en contacto con su médico si tiene cualquier pregunta.
Conducción y uso de máquinas
Se prevé que la influencia de EVENITY sobre la capacidad para conducir o usar máquinas sea nula o insignificante.
EVENITY contiene sodio y polisorbato 20
Este medicamento contiene 0,070 mg de polisorbato 20 en cada pluma precargada. Los polisorbatos pueden provocar reacciones alérgicas. Informe a su médico si tiene alguna alergia.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis, esto es, esencialmente exento de sodio.
3. Cómo usar EVENITY
El tratamiento con EVENITY será iniciado y supervisado por médicos especializados con experiencia en el tratamiento de la osteoporosis. Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte a su médico.
Solo una persona con la formación adecuada debe poner la inyección.
Cuánto utilizar
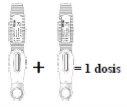
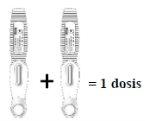
- La dosis recomendada de EVENITY es de 210 mg.
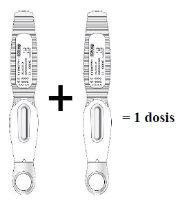
- Dado que una pluma precargada contiene 105 mg del principio activo romosozumab en 1,17 ml de solución (90 mg/ml), deben usarse 2 plumas precargadas para cada dosis. La segunda inyección debe administrarse inmediatamente después de la primera pero en un lugar de la inyección distinto.
- Esto debe hacerse una vez al mes durante 12 meses.
Cómo usar
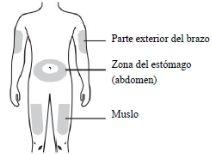
- EVENITY debe inyectarse bajo la piel (inyección por vía subcutánea).
- EVENITY deberá inyectarse en la zona del estómago (abdomen) o el muslo. La parte exterior del brazo también se puede usar como lugar de la inyección, pero solo si otra persona le pone la inyección.
- Si se prevé usar la misma zona de la inyección para la segunda inyección, deberá usarse un punto distinto.
- EVENITY no deberá inyectarse en zonas en las que la piel esté dolorida, con hematomas, roja o endurecida.
Es importante que lea las Instrucciones de usopara obtener información sobre cómo usar la pluma precargada EVENITY.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si usa más EVENITY del que debe
Si ha usado más EVENITY del que debe por error, debe ponerse en contacto con su médico o farmacéutico.
Si olvidó o no puede usar EVENITY en su momento habitual
Si omite una dosis de EVENITY, póngase en contacto con su médico lo antes posible para programar otra dosis. A partir de entonces, la siguiente dosis debe administrarse al menos un mes después de la fecha de la última dosis.
Si interrumpe el tratamiento con EVENITY
Si se está planteando interrumpir el tratamiento con EVENITY, coméntelo con su médico. Su médico le indicará durante cuánto tiempo debe recibir tratamiento con EVENITY.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Comente con su médico la necesidad de cambiar a otro tratamiento para la osteoporosis después del final de su tratamiento con EVENITY.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Busque inmediatamente atención médicasi presenta cualquiera de los posibles síntomas de infarto de miocardioo accidente cerebrovascular(poco frecuentes: pueden afectar a hasta 1 de cada 100 personas) que se indican a continuación:
- dolor en el pecho, dificultad para respirar;
- dolor de cabeza, entumecimiento o debilidad en el rostro, los brazos o las piernas, dificultad para hablar, cambios en la visión, pérdida de equilibrio.
Busque inmediatamente atención médicasi presenta los siguientes síntomas de reacción alérgica grave(raros: pueden afectar a hasta 1 de cada 1.000 personas) que se indican a continuación:
- hinchazón del rostro, la boca, la garganta, las manos, los pies, los tobillos,la parte inferior de las piernas (angioedema), o ronchas;
- erupción cutánea aguda que muestra múltiples puntos rojos/rosas redondos con una ampolla o una costra en el centro (eritema multiforme);
- dificultad para tragar o respirar.
Avise a su médicosi observa los síntomas de niveles bajos de calcioen la sangre (hipocalcemia)(poco frecuentes: pueden afectar a hasta 1 de cada 100 personas) que se indican a continuación:
- espasmos, tirones o calambres musculares;
- entumecimiento u hormigueo en los dedos de las manos o de los pies, o alrededor de la boca.
Consulte también la sección 2 “Qué necesita saber antes de empezar a usar EVENITY”.
Otros efectos adversos pueden incluir:
Efectos adversos muy frecuentes(que pueden afectar a más de 1 de cada 10 personas):
- Resfriado común;
- Dolor articular.
Efectos adversos frecuentes(que pueden afectar a hasta 1 de cada 10 personas):
- Exantema, inflamación de la piel;
- Dolor de cabeza;
- Inflamación de los senos paranasales;
- Dolor de cuello;
- Espasmos musculares;
- Enrojecimiento o dolor en torno al lugar donde se ha administrado la inyección.
Efectos adversos poco frecuentes(que pueden afectar a hasta 1 de cada 100 personas):
- Ronchas (urticaria);
- Cataratas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de los efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de EVENITY
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de “EXP/CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Una vez que saque la caja con las plumas precargadas de la nevera para su uso, no deberá volver a ponerla en ella, pero puede mantenerla a temperatura ambiente (hasta 25 °C) durante 30 días. Si no se usa dentro de este período, el producto debe desecharse.
Mantener la pluma precargada en el embalaje exterior para protegerla de la luz.
Compruebe visualmente la solución. No debe usar la solución si ha cambiado de color, está turbia o contiene escamas o partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de EVENITY
- El principio activo es romosozumab. Cada pluma precargada contiene 105 mg de romosozumab en 1,17 ml de solución (90 mg/ml).
- Los demás componentes son acetato de calcio, ácido acético glacial, hidróxido de sodio (para el ajuste del pH), sacarosa, polisorbato 20 y agua para preparaciones inyectables. Ver sección 2 “EVENITY contiene sodio”.
Aspecto del producto y contenido del envase
EVENITY es una solución inyectable de clara a opalescente, de incolora a amarillenta que se presenta en una pluma precargada desechable de un solo uso. La jeringa en el interior de la pluma está hecha de plástico y tiene una aguja de acero inoxidable.
Envase con 2 plumas precargadas.
Envase múltiple con 6 (3 envases de 2) plumas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
UCB Pharma S.A.,
Allée de la Recherche 60,
B-1070 Bruselas, Bélgica
Responsable de la fabricación
Amgen Europe B.V., Minervum 7061, 4817 ZK Breda, Países Bajos
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien UCB Pharma SA/NV Tél/Tel: + 32 / (0)2 559 92 00 | Lietuva UCB Pharma Oy Finland Tel: + 358 9 2514 4221 |
| Luxembourg/Luxemburg UCB Pharma SA/NV Tél/Tel: + 32 / (0)2 559 92 00 |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Nederland UCB Pharma B.V. Tel.: + 31 / (0)76-573 11 40 |
Eesti UCB Pharma Oy Finland Tel: + 358 9 2514 4221 | Norge UCB Nordic A/S Tlf: + 45 / 32 46 24 00 |
Ελλ?δα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 / 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Simi: + 354 535 7000 | Slovenská republika UCB s.r.o., organizacná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κ?προς Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 34 74 40 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 29 49 00 |
Latvija UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Somija) |
Fecha de la última revisión de este prospecto: MM/AAAA.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
De la vuelta a la página para ver las instrucciones de uso
--------------------------------------------------------------------------------------------------------------------
INSTRUCCIONES DE USO PARA LA INYECCIÓN DE EVENITY MEDIANTE UNA PLUMA PRECARGADA
Inyectar dos plumas precargadas, una inmediatamente después de la otra, para administrar una dosis completa
Las instrucciones que se incluyen a continuación explican cómo usar la pluma precargada para inyectar EVENITY.
- Lea detenidamente estas instrucciones y sígalas paso a paso.
- Si tiene alguna pregunta o no está segura sobre el procedimiento de inyección, póngase en contacto con un médico o farmacéutico.
- Es importante asegurarse de que únicamente una persona con la formación adecuada administra la inyección.
- La pluma precargada también se denomina el “medicamento”.
Guía de las piezas: pluma precargada
| Lea esto antes de inyectar el medicamento. El médico que le atiende le ha recetado una dosis de 210 mg cada mes: Para recibir la dosis completa,deberán inyectarse dos plumas precargadas con 105 mg cada una, una inmediatamente después de la otra. |
|
Paso 1: Preparar
A
- Saque la caja que contiene las dos plumas precargadas de la nevera.
- Sus plumas precargadas deberán dejarse fuera de la nevera para que alcancen la temperaturaambiente (hasta 25 °C) durante al menos 30 minutosantes de la inyección (no deben calentarse de ningún otro modo). Esto hará que la inyección sea más cómoda.
- Abra la caja y recopile todos los materiales que necesita para la inyección (según se enumeran enel Paso B)
- Lávese bien las manos.
- Levante las plumas precargadas directamente hacia arriba para sacarlas de la caja. No retire lostapones blancos de las plumas todavía.
- No agite las plumas precargadas.
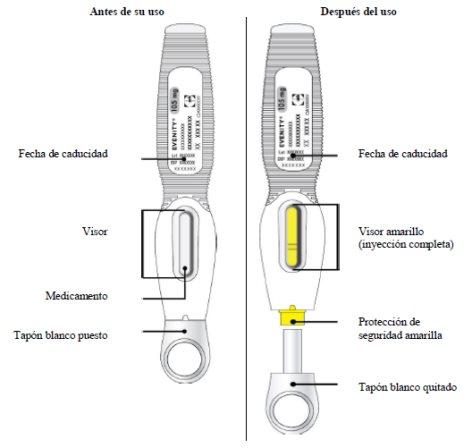
- Compruebe el medicamento a través del visor. El medicamento deberá ser una solución entreclara y opalescente, de incolora a amarillenta.
- No debe usarse la pluma precargada si la solución ha cambiado de color, está turbia o contieneescamas o partículas.
- Es posible que se vean burbujas de aire. La inyección por vía subcutánea (bajo la piel) de unasolución que contenga burbujas de aire es inocua.
- No use la pluma precargada si:
- Se ha caído;
- El tapón blanco no está o no está firmemente acoplado;
- El precinto no está o está roto o si cualquier parte aparece agrietada o rota.
En estos casos, use una pluma nueva y contacte con su médico lo antes posible.
BEn una superficie de trabajo limpia y bien iluminada, coloque:
|
|
CPrepare y limpie la piel en la que va a inyectar el medicamento. Puede escoger entre:
|
|
- La segunda inyección debe administrarse en un lugar distinto al de la primera inyección. Si desea usar el mismo lugar de la inyección, compruebe que no sea el mismo punto de inyección exactamente.
- No debe realizar la inyección en las zonas en las que la piel está dolorida, con hematomas, enrojecida, endurecida, con cicatrices o estrías, o muestra lesiones o manchas gruesas, enrojecidas o con escamas.
- Limpie con una toallita con alcohol la zona en la que va a inyectarse. Deje que se seque la piel antes de la inyección.
- No vuelva a tocar esta zona antes de la inyección.
Paso 2: Prepárese
D |
|
- No tuerza ni doble el tapón blanco.
- Deseche el tapón blanco en el recipiente para residuos especiales. No vuelva a colocar el tapón blanco en la pluma precargada.
- Aunque está oculta a la vista, la punta de la aguja está ahora al descubierto. No intente tocar la aguja, dado que podría activar la pluma precargada. Es normal ver una gota de líquido en la punta de la aguja (dentro de la protección de seguridad amarilla).
EEstire o apriete el lugar de la inyección para crear una superficie firme.
Estire
- Estire bien la piel moviendo el pulgar y los dedos en direcciones opuestas, para crear una zona de 5 cm de ancho.
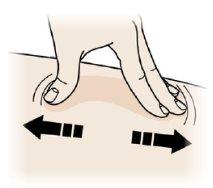
O
Apriete
- Apriete firmemente la piel entre el pulgar y los dedos para crear una zona de aproximadamente 5 cm de ancho
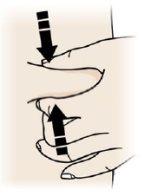
- Importante:Mantenga la piel estirada o apretada durante la inyección.
Paso 3: Ponga la inyección
F |
|
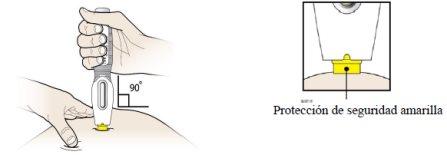
G |
|
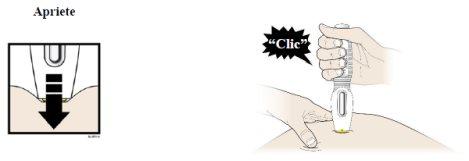
H |
|
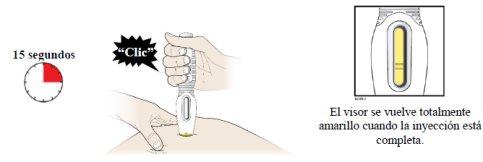
- Ahora puede retirar la pluma precargada tirando con cuidado de ella directamente hacia fuera alejándola de la piel.
- Importante:Cuando retire la pluma precargada, si el visor no se ha vuelto totalmente amarillo o si parece que continúa inyectándose el medicamento, esto indica que no se ha administrado la dosis completa. Debe informar a su profesional sanitario lo antes posible.
- Después de retirar la pluma precargada de la piel, la aguja quedará cubierta de forma automática. No intente tocar la aguja.
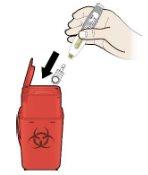
Paso 4: Desechar
I |
|
|
|
Paso 5: Examine el lugar de la inyección
J | Si hay sangre, use un trozo de algodón o de gasa y apriete un poco sobre el lugar de la inyección durante algunos segundos. No frote el lugar de la inyección. Puede cubrirse el lugar de la inyección con una tirita, si es necesario. |
Paso 6: Repita el proceso para poner la segunda inyección y conseguir la dosis completa
K | Repita todos los pasos a partir del paso C con la segunda pluma precargada para inyectar la dosis completa. La segunda inyección debe administrarse en un lugar distinto al de la primera inyección. Si desea usar el mismo lugar de la inyección, compruebe que no sea exactamente en el mismo punto de inyección. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EVENITY 105 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 120 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE, 120 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere recetaForma farmacéutica: INYECTABLE, 60 mgPrincipio activo: DenosumabFabricante: Fresenius Kabi Deutschland GmbhRequiere receta
Médicos online para EVENITY 105 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EVENITY 105 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes