
EPINITRIL 10 mg/24 H PARCHES TRANSDERMICOS


Cómo usar EPINITRIL 10 mg/24 H PARCHES TRANSDERMICOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Epinitril 10 mg/24 h parche transdérmico
Trinitrato de glicerilo
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Epinitril y para qué se utiliza
- Qué necesita saber antes de empezar a usar Epinitril
- Como usar Epinitril
- Posibles efectos adversos
- Conservación de Epinitril
- Contenido del envase e información adicional
1. Qué es EPINITRIL y para qué se utiliza
Los parches de Epinitril contienen el principio activo trinitrato de glicerilo, un vasodilatador que se usa en las enfermedades cardíacas y pertenece a un grupo de fármacos denominados nitratos orgánicos.
Los parches de Epinitril se aplican a la piel y el principio activo pasará entonces de forma continua a través de la piel y al organismo.
Epinitril está indicado para el tratamiento preventivo de la angina de pecho tomado solo o en combinación con otro tratamiento antianginoso.
La angina normalmente se presenta como un dolor u opresión en el pecho, aunque puede notarse en el cuello o el brazo.
El dolor aparece cuando el corazón no está suficientemente oxigenado. Epinitril no está indicado para el tratamiento de los ataques agudos. Utilice su comprimido o spray sublingual para el tratamiento de los ataques agudos.
Los parches de Epinitril son para uso externo sólo.
2. Qué necesita saber antes de empezar a usar EPINITRIL
No useEpinitril:
- si es alérgico (hipersensible) al trinitrato de glicerilo, nitratos orgánicos relacionados o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si tiene o ha sufrido recientemente un colapso asociado a hipotensión;
- si sufre dolor de cabeza, vómitos o convulsiones asociadas a un aumento de la presión intracraneal, incluidas las causadas por traumatismo en la cabeza;
- si sufre una insuficiencia cardíaca por obstrucción, por ejemplo, en presencia de un estrechamiento del orificio aórtico o del orificio auriculoventricular del corazón (estenosis aórtica o estenosis mitral, respectivamente) o un engrosamiento fibrótico de la delgada membrana que recubre al corazón (pericarditis constrictiva);
- si está tomando medicamentos para el tratamiento de la disfunción eréctil (p.ej., sildenafilo o cualquier otro inhibidor de la PDE-5). Los nitratos no deben administrarse a los pacientes tratados con sildenafilo o cualquier otro medicamento usado para tratar la disfunción eréctil. Los pacientes tratados actualmente con nitratos no deben tomar sildenafilo ni ningún otro medicamento para el tratamiento de la disfunción eréctil. La combinación de un nitrato con sildenafilo o cualquier otro inhibidor de la PDE-5 puede provocar una reducción profunda y repentina de la tensión arterial, que puede producir desmayos, pérdida de conocimiento o incluso un ataque al corazón (véase también “Uso de otros medicamentos”);
- si está tomando medicamentos con riociguat, un estimulador de la guanilato ciclasa soluble,
- si sufre una grave bajada de tensión arterial (presión arterial menor de 90 mmHg),
- si sufre una bajada grave del volumen de sangre corporal debido a pérdida de sangre o de fluidos corporales (hipovolemia severa),
- si sufre una anemia grave,
- si sufre una retención de líquidos tóxica en los pulmones (edema pulmonar tóxico).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Epinitril:
- si se retira del tratamiento. La retirada del tratamiento con Epinitril debe ser gradual, sustituyéndose por dosis decrecientes de nitratos orales de acción prolongada;
- si debe someter a resonancia magnética, estimulación eléctrica del corazón para re-establecer el ritmo cardíaco normal (desfibrilación o cardioversión) y antes del tratamiento con calor (diatermia). Retire los parches de Epinitril antes de someterse a estos tratamientos;
- si sufre o ha sufrido recientemente un ataque al corazón (infarto de miocardio) o si aparecen rápidamente síntomas de insuficiencia cardíaca (insuficiencia cardíaca aguda) como dificultad para respirar, sensación de mucho cansancio, hinchazón de piernas. Su médico puede solicitar exámenes de laboratorio de sus funciones cardiovasculares;
- si sufre una grave hipotensión durante el tratamiento con Epinitril, debe considerarse retirar el parche. En caso de colapso o shock, el parche debe retirarse;
- si experimenta un dolor en el pecho (ataque agudo de angina) o si su corazón no bombea suficiente sangre y oxígeno (angina inestable) o en caso de ataque de corazón (infarto de miocardio), no debe usarse Epinitril como un tratamiento inmediato;
- si sufre un fuerte dolor de cabeza o presión arterial baja (hipotensión) anómala. Esto puede ocurrir si la dosis inicial es demasiado alta. Es aconsejable aumentar la dosis progresivamente hasta lograr el efecto óptimo;
- si está tomando otros nitratos o trinitrato de glicerilo sublingual, porque su organismo puede desarrollar una resistencia a los efectos de estas sustancias tras la exposición repetida (tolerancia cruzada);
- si sufre o ha sufrido una presión arterial baja anómala provocada por el trinitrato de glicerilo. En este caso, puede sufrir una frecuencia cardíaca baja (bradicardia paradójica) y un aumento de la angina;
- si sufre una enfermedad del nervio óptico (glaucoma de ángulo cerrado);
- si tiene una oxigenación insuficiente de la sangre (hipoxemia) por una anemia grave o una enfermedad pulmonar o un menor flujo sanguíneo al corazón (insuficiencia cardíaca isquémica); los pacientes en estos problemas médicos pueden sufrir un desequilibrio en la relación ventilación/perfusión que es un índice de la función respiratoria. En estos pacientes, el trinitrato de glicerilo puede empeorar este desequilibrio y causar una disminución de la oxigenación de la sangre;
- si la angina ha sido provocada por un engrosamiento del corazón (miocardiopatía hipertrófica). Los nitratos pueden empeorar este tipo de angina;
- si sufre una mayor frecuencia de ataques de angina durante los períodos sin parche. Puede que su médico quiera volver a evaluar su enfermedad coronaria y considere una adaptación del tratamiento.
- el tratamiento debe de ser suspendido y debe consultar con el médico si experimenta fenómenos de sensibilización de la piel (picor, quemazón, inflamación).
Uso de Epinitril con otros medicamentos
La administración concomitante de medicamentos para el tratamiento de la disfunción eréctil (p.ej., sildenafilo o cualquier otro inhibidor de PDE-5) potencia los efectos reductores de la tensión arterial de los nitratos y, por tanto, debe evitarse (véase también “No use Epinitril”).
Debe evitarse el tratamiento simultáneo con riociguat, un estimulador de la guanilato ciclasa soluble, dado que el uso simultáneo puede causar hipotensión (véase también “No use Epinitril”)
El tratamiento concomitante con
- medicamentos usados para disminuir la presión arterial, tales como antagonistas del calcio, inhibidores de la ECA (para el tratamiento de insuficiencia cardiaca congestiva), beta-bloqueantes(usados para prevenir las arritmias cardiacas), diuréticos, (incrementan la excreción de agua desde el cuerpo), y otros antihipertensivos,
- antidepresivos tricíclicos (medicamentos usados para el tratamiento de la depresión),
- neurolépticos (medicamentos usados para prevenir la psicosis),
- tranquilizantes mayores (sedantes), así como el consumo de alcohol y en asociación con amifostina(medicamento citoprotector en quimioterapia y radioterapia),
- ácido acetilsalicílico (un AINE),
puede potenciar los efectos reductores de la presión arterial de Epinitril.
El tratamiento simultáneo con dihidroergotamina puede disminuir el efecto de Epinitril.
Medicamentos antiinflamatorios no esteroideos, excepto el ácido acetilsalicílico, pueden disminuir la respuesta terapéutica de Epinitril.
Comunique a su médico o farmacéutico que está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Epinitril no debe utilizarse durante el embarazo, especialmente durante los tres primeros meses a no ser que su médico se lo haya indicado.
Puesto que casi no hay información sobre si el trinitrato de glicerilo se excreta en la leche materna, no se puede excluir un riesgo en la lactancia. Su doctor evaluará si debe interrumpir la lactancia o el tratamiento con Epinitril.
No hay datos del efecto de Epinitril sobre la fertilidad en humanos.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Al comienzo sobre todo del tratamiento, o durante el ajuste de la dosis, Epinitril puede influir sobre la capacidad de conducir o usar maquinaria, reducir la capacidad de reacción o producirse raramente hipotensión ortostática y mareo, así como excepcionalmente, un síncope tras sobredosificación.
Si usted experimenta estos efectos, debe abstenerse de conducir o utilizar máquinas.
3. Cómo usar EPINITRIL
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es un parche de Epinitril una vez al día. Aplique el parche en la piel cuidadosamente y manténgalo durante 12-16 horas. Luego, quítese el parche y mantenga un período sin parche durante las 8-12 horas restantes.
Debe cambiar su parche de Epinitril de acuerdo con las instrucciones dadas por su médico. Su médico le dirá cuánto tiempo debe mantener el parche en la piel y la duración del intervalo sin parche.
Uso en niños y adolescentes
Epinitril no debe usarse en niños ni adolescentes menores de 18 años.
Durante cuánto tiempo debe usar Epinitril
El tratamiento con Epinitril podrá continuar durante varios años; sin embargo, su médico querrá verle periódicamente para decidir si continúa con el tratamiento o modifica la pauta terapéutica.
Cómo ponerse el parche
Aplíquese el parche en la piel limpia y seca, pero no encima de cortes, manchas ni defectos ni en zonas donde acabe de aplicarse crema, hidratante o talco. Se recomienda aplicar Epinitril parches transdérmicos en la piel del pecho (véase la Figura 1) o la parte superior externa del brazo, libre de enrojecimiento o irritación, y rotar los puntos de aplicación. Si es necesario puede rasurarse la zona adecuada. Se evitarán las zonas que formen pliegues o estén sujetas a roces durante el movimiento.
Figura 1
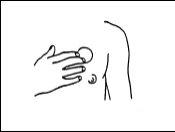
No se aplique dos parches consecutivos en el mismo lugar.
Se aplicará un parche de Epinitril en la piel en cuanto lo saque del sobre, de la forma siguiente:
(I) Rompa el sobre por la línea de puntos.
No utilice tijeras (véase la Figura 2).
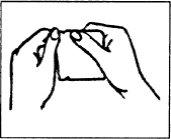
Figura 2
(II) Sostenga el parche entre el pulgar y el
dedo índice por la etiqueta que se quita
(véase la Figura 3).
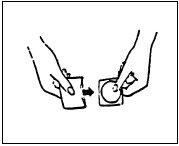
Figura 3
(III) Quite el recubrimiento protector con la otra
mano (véase la Figura 4). No toque el lado
pegajoso del parche; de lo contrario, no se pegará bien.
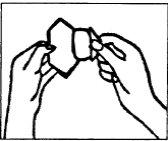
Figura 4
(IV) Aplíquese la parte abierta del parche en la piel
y quite la parte restante del recubrimiento protector.
Apriete con firmeza durante unos 10 segundos sobre
la superficie total del parche. Pase los dedos por los
bordes para asegurarse de que se pegan bien.
Lávese las manos antes y después de aplicar Epinitril.
Para quitar un parche, levante el borde y tire suavemente del parche hasta que salga. Después de usar el parche, dóblelo por la mitad, con la parte pegajosa hacia adentro y tírelo a la basura, donde los niños no puedan cogerlo.
¿Qué hacer si el parche se cae?
Si Epinitril se aplica correctamente, es muy improbable que el parche se caiga. Sin embargo, si el parche se cae, sustitúyalo por uno nuevo y luego cambie de nuevo el parche de la forma habitual según su calendario original.
Si usa más Epinitril del que debe
Si se administra dosis elevadas de trinitrato de glicerilo puede experimentar hipotensión grave y taquicardia refleja o colapso y síncope, así como alteración de la hemoglobina (metahemoglobinemia).Si se aplican demasiados parches de una sola vez, deben quitarse los parches cuidadosamente y lavar la piel de debajo minuciosamente para reducir la absorción. En caso de que experimente hipotensión o colapso, se aconseja elevar las piernas del paciente o si es necesario, aplicar un vendaje compresivo en las piernas.
En caso de intoxicación debe informar a su médico de inmediato, contactar inmediatamente con el servicio de urgencias más cercano o llamar al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad usada. Muestre a su médico el medicamento o el envase vacío.
Si olvidó cambiar el parche
Si olvida cambiar el parche en el momento correcto, debe sustituirlo lo antes posible y luego seguir el calendario original para aplicar el parche siguiente.
Si interrumpe el tratamiento con Epinitril
Al interrumpir el tratamiento con Epinitril, puede volver a experimentar ataques de angina.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Epinitril puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado los siguientes efectos secundarios:
Efectos adversos muy frecuentes (se producen en más de 1 paciente de cada 10):
- Náuseas.
- Vómitos.
Efectos adversos frecuentes (se producen en más de 1 y menos de 10 pacientes de cada 100):
- Dolor de cabeza.
Efectos adversos poco frecuentes (se producen en más de 1 y menos de 10 pacientes de cada 1.000):
- Inflamación en la zona de contacto de la piel (dermatitis de contacto).
- Enrojecimiento e irritación en la zona de aplicación del parche.
- Picor.
- Sensación de quemazón.
Efectos adversos raros (se producen en más de 1 y menos de 10 pacientes de cada 10.000):
- Taquicardia.
- Hipotensión ortostática (descenso de la tensión arterial al levantarse), pueden ser descritos como episodios transitorios de mareo.
- Enrojecimiento de la piel.
- Aumento de la frecuencia cardíaca.
Efectos adversos muy raros (se producen en menos de 1 de cada 10.000 pacientes):
- Mareo.
- Síncope.
Efectos adversos de frecuencia no conocida:
- Trastornos cardíacos( palpitación).
- Erupción cutánea generalizada (rash generalizado).
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
.
5. Conservación de EPINITRIL
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25°C. Epinitril debe guardarse en su sobre intacto.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y el sobre después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Epinitril
El principio activo de los parches de Epinitril es trinitrato de glicerilo y están disponibles en tres concentraciones: Epiniril 5mg/24h, 10 mg/24h y 15 mg/24h.
Epinitril 10 mg/24 h: contiene 31,37 mg del principio activo trinitrato de glicerilo y libera alrededor de 10 mg de trinitrato de glicerilo al día (0,4 mg/h); la zona de liberación del parche es de 12,75 cm2. El código de identificación impreso en la lámina de soporte es NR10.
Los demás componentes son una sustancia adhesiva (copolímero acrilato-vinilacetato), un taquificante (ftalato de hidroabietilo) y un reticulante (polibutiltitanato), que se han extendido junto con el principio activo en una lámina de soporte (lámina de polipropileno lacada). La capa adhesiva está cubierta por un recubrimiento protector de aluminio y silicona en los dos lados que se quita antes del uso.
Aspecto del producto y contenido del envase
Epinitril son parches transdérmicos con la parte posterior adhesiva. Cada parche está sellado de forma individual en un sobre protector.
Tamaños de envase: 15 y 30 parches. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsable de la fabricación
ROTTAPHARM Ltd.
Damastown, Industrial Park, Mulhuddart
Dublín 15
Irlanda.
O
LTS Lohmann Therapie Systeme AG
Lohmannstraße 2
56626 Andernach
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
España
Fecha de la última revisión de este prospecto:diciembre 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia15.42 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EPINITRIL 10 mg/24 H PARCHES TRANSDERMICOSForma farmacéutica: PARCHE TRANSDERMICO, 37,4 mg nitroglicerinaPrincipio activo: Trinitrato de gliceriloFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 18,7 mg nitroglicerinaPrincipio activo: Trinitrato de gliceriloFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 31,37 mgPrincipio activo: Trinitrato de gliceriloFabricante: Casen Recordati S.L.Requiere receta
Médicos online para EPINITRIL 10 mg/24 H PARCHES TRANSDERMICOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EPINITRIL 10 mg/24 H PARCHES TRANSDERMICOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











