
EBASTINE RATIOPHARM 20 mg ORALLY DISINTEGRATING TABLETS

How to use EBASTINE RATIOPHARM 20 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ebastineratiopharm20 mg Orodispersible Tablets EFG
Ebastine
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Ebastine ratiopharm and what is it used for
- What you need to know before you take Ebastine ratiopharm
- How to take Ebastine ratiopharm
- Possible side effects
- Storage of Ebastine ratiopharm
Contents of the pack and other information
1. What is Ebastine ratiopharm and what is it used for
Ebastine is an antihistamine that helps to relieve the symptoms of allergy such as sneezing, runny nose, itchy eyes and itchy skin rashes.
In adults and children of 12 years and older, Ebastine ratiopharm is used to relieve the symptoms of seasonal and perennial allergic rhinitis, including cases with allergic conjunctivitis.
2. What you need to know before you take Ebastine ratiopharm
Do not take Ebastine ratiopharm
If you are allergic to ebastine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before taking Ebastine ratiopharm if:
- you have low levels of potassium in your blood.
- you are already taking certain antibiotics or medicines used to treat fungal infections: see the section "Taking Ebastine ratiopharm with other medicines" below.
- you have severe liver function disorders (liver failure).
Ebastine may cause dry mouth. Therefore, during prolonged treatment with ebastine, it is important to maintain good oral hygiene (you should brush your teeth twice a day with a fluoride toothpaste) to reduce the risk of tooth decay.
Children and adolescents
This medicine should only be taken by children from 12 years of age. Do not give to children under 12 years of age since the safety and efficacy have not been established in this age group.
Taking Ebastine ratiopharm with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Taking Ebastine ratiopharm at the same time as erythromycin (an antibiotic) or ketoconazole, itraconazole (active substances for the treatment of fungal infections), may lead to higher levels of ebastine in the blood.
Taking ebastine at the same time as rifampicin (a medicine against tuberculosis) may lead to lower levels of ebastine in the blood, resulting in a reduction of the effects.
It is not recommended to use Ebastine ratiopharm at the same time as clarithromycin or josamycin (antibiotics).
Taking Ebastine ratiopharm with food and drinks
You can take Ebastine ratiopharm with or without food.
Pregnancy and breast-feeding
Up to now, the existing experience regarding safety for the fetus in humans is limited. For this reason, you should only take this medicine during pregnancy if your doctor considers that the expected benefit outweighs the possible risks.
Do not take Ebastine ratiopharm if you are breast-feeding since it is not known whether the active substance passes into breast milk.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Most patients treated with Ebastine ratiopharm can drive or perform other activities that require a good reaction ability. However, as with other medicines, you should check your individual reaction after taking Ebastine ratiopharm before driving or performing complex activities, since some patients experience drowsiness or dizziness.
Ebastine ratiopharm contains aspartame
This medicine contains 5 mg of aspartame in each tablet.
Aspartame is a source of phenylalanine, which may be harmful for people with phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body is unable to eliminate it properly.
Ebastine ratiopharm contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
Ebastine ratiopharm contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; this is essentially "sodium-free".
3. How to take Ebastine ratiopharm
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
The recommended dose is:
Indication | Age | Dose |
Allergic rhinitis In case of intense symptoms | Children of 12 years and older and adults | One Ebastine ratiopharm 10 mg tablet (10 mg of ebastine) per day Two Ebastine ratiopharm 10 mg tablets or one Ebastine ratiopharm 20 mg tablet (20 mg of ebastine) per day |
Urticaria | Adults over 18 years | One Ebastine ratiopharm 10 mg tablet (10 mg of ebastine) per day |
In patients with renal insufficiency, no dose adjustment is necessary.
In patients with mild to moderate liver insufficiency, no dose adjustment is necessary.
There is no experience with doses above 10 mg in patients with severe liver insufficiency, so these patients should not take more than 10 mg per day.
Do not push the tablet out of the blister, as this will damage it.
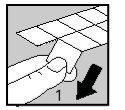
Each strip contains tablets separated by perforations in blisters. Separate one blister with its tablet along the dotted line (Figure 1).
Carefully peel off the protective film, starting from the corner indicated by the arrow (Figures 2 and 3).
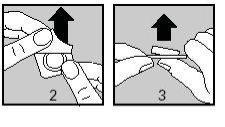
Keep your hands dry and take the tablet from the strip.
Place the tablet on the tongue, where it will disperse. No water or other liquid is needed.
You can take Ebastine ratiopharm with or without food.
Your doctor will decide the duration of the treatment.
If you take more Ebastine ratiopharm than you should
There is no specific antidote for the active substance ebastine.
If you suspect an overdose with Ebastine ratiopharm, inform your doctor. Depending on the severity of the poisoning, he/she will take the necessary measures (monitoring of the body's vital functions, including ECG monitoring for at least 48 hours, symptomatic treatment, and gastric lavage), if necessary.
If you have taken a dose higher than that prescribed by your doctor or in case of overdose, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used.
If you forget to take Ebastine ratiopharm
Do not take a double dose to make up for forgotten doses.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Ebastine ratiopharm and contact your doctor immediately or go to the nearest hospital if you experience the following:
- Severe allergic reaction that causes swelling of the face, tongue, or throat and may cause difficulty swallowing or breathing.
Other side effects include:
Very common (may affect more than 1 in 10 people)
- Headache
Common (may affect up to 1 in 10 people):
- Drowsiness.
- Dry mouth.
Uncommon (may affect up to 1 in 100 people):
- Sore throat (pharyngitis), runny nose (rhinitis), nosebleeds.
- Abdominal pain, nausea, indigestion.
- Weakness (asthenia), dizziness, insomnia.
Rare (may affect up to 1 in 10,000 people):
- Nervousness, insomnia.
- Abnormalities or decreased sense of touch.
- Touch disorders
- Palpitations, rapid heartbeat.
- Abdominal pain, nausea, indigestion
- Vomiting.
- Abnormal liver function test.
- Liver inflammation
- Bile flow disorders causing itching, yellow eyes, and skin
- Rash, annoying rash, skin inflammation.
- Menstrual disorders.
- Edema (water retention in the tissues), weakness (asthenia)
- Dizziness.
Very rare (may affect up to 1 in 10,000 people)
- Hives, eczema
- Painful menstruation
Frequency not known (cannot be estimated from the available data)
- Weight gain,
- Increased appetite.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Agency's website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ebastine ratiopharm
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month.
Store in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Ebastine ratiopharm
The active substance is ebastine.
Each orodispersible tablet contains 20 mg of ebastine.
The other ingredients are: microcrystalline cellulose, lactose monohydrate, corn starch, sodium croscarmellose, aspartame (E-951), peppermint flavor, colloidal anhydrous silica, and magnesium stearate.
Appearance and packaging
Orodispersible tablet.
White, biconvex, round tablets, engraved with "E20" on one side and smooth on the other.
Available pack sizes: 10, 15, 20, 30, 40, 50, 98, and 100 orodispersible tablets.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Teva Pharma, S.L.U.
C / Anabel Segura 11, Edificio Albatros B, 1st floor
Alcobendas 28108 Madrid (Spain)
Manufacturer
Teva Pharmaceutical Works Private Limited Company
Pallagi út 13, 4042 Debrecen
Hungary
This medicine is authorised in the Member States of the European Economic Area under the following names:
ES: Ebastine ratiopharm 20 mg orodispersible tablets EFG
IT: Ebakest 20 mg orodispersible tablets
SE: Ebateva 20 mg orodispersible tablets
Date of last revision of this leaflet: September 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price8.79 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EBASTINE RATIOPHARM 20 mg ORALLY DISINTEGRATING TABLETSDosage form: TABLET, 10 mg ebastineActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 10 mgActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not requiredDosage form: TABLET, 20 mg ebastineActive substance: ebastineManufacturer: Laboratorios Almirall S.L.Prescription not required
Online doctors for EBASTINE RATIOPHARM 20 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about EBASTINE RATIOPHARM 20 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















