
DUPIXENT 300 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar DUPIXENT 300 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Dupixent 300 mg solución inyectable en jeringa precargada
dupilumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Dupixent y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dupixent
- Cómo usar Dupixent
- Posibles efectos adversos
- Conservación de Dupixent
- Contenido del envase e información adicional
1. Qué es Dupixent y para qué se utiliza
Qué es Dupixent
Dupixent contiene el principio activo dupilumab.
Dupilumab es un anticuerpo monoclonal (un tipo de proteína especializada) que bloquea la acción de las proteínas llamadas interleucinas (IL)-4 e IL-13. Ambas juegan un papel clave en la aparición de los signos y síntomas de la dermatitis atópica, el asma, la rinosinusitis crónica con poliposis nasal (RSCcPN), el prurigo nodular (PN), la esofagitis eosinofílica (EEo) y la enfermedad pulmonar obstructiva crónica (EPOC).
Para qué se utiliza
Dupixent se usa para tratar adultos y adolescentes a partir de 12 años con dermatitis atópica de moderada a grave, también conocida como eccema atópico. Dupixent se usa también para tratar niños de 6 meses a 11 años con dermatitis atópica grave. Dupixent se puede utilizar con medicamentos para el eccema que se aplican en la piel o se puede utilizar solo.
Dupixent se usa también, junto con otros medicamentos para el asma, para el tratamiento de mantenimiento del asma grave en adultos, adolescentes y niños a partir de 6 años cuya asma no se controla con su medicación actual (p. ej. corticosteroides).
Dupixent se usa también, junto con otros medicamentos, para el tratamiento de mantenimiento de la RSCcPN en adultos cuya enfermedad no se controla con su medicación actual para la RSCcPN. Dupixent también puede reducir la necesidad de cirugía y la necesidad del uso de corticosteroides sistémicos.
Dupixent se usa también para tratar adultos con prurigo nodular (PN) de moderado a grave, también conocido como prurigo crónico nodular (PCN). Dupixent se puede utilizar con medicamentos para el PN que se aplican en la piel o se puede utilizar solo.
Dupixent se usa también para tratar adultos, adolescentes y niños a partir de 1 año, con un peso mínimo de 15 kg, con esofagitis eosinofílica (EEo).
Dupixent se usa también, junto con otros medicamentos, para el tratamiento de mantenimiento de la enfermedad pulmonar obstructiva crónica (EPOC) en adultos con EPOC no controlada.
Cómo funciona Dupixent
El uso de Dupixent para la dermatitis atópica (eccema atópico) puede mejorar la enfermedad de su piel y reducir el picor. Dupixent también ha demostrado una mejoría de los síntomas del dolor, ansiedad y depresión asociados con la dermatitis atópica. Además, Dupixent ayuda a mejorar el trastorno del sueño y su calidad de vida en general.
Dupixent ayuda a prevenir los ataques de asma graves (exacerbaciones) y puede mejorar su capacidad respiratoria. Dupixent también puede ayudar a reducir la cantidad de otro grupo de medicamentos que usted necesita para controlar su asma, denominados corticosteroides orales, mientras que previene los ataques de asma graves y mejora su capacidad respiratoria.
Dupixent ayuda a prevenir los ataques de EPOC moderados o graves (exacerbaciones) y puede mejorar su capacidad respiratoria. Dupixent también puede ayudar a mejorar los síntomas generales de la EPOC.
2. Qué necesita saber antes de empezar a usar Dupixent
No use Dupixent
- si es alérgico a dupilumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si piensa que puede ser alérgico, o no está seguro, consulte a su médico, farmacéutico o enfermero antes de utilizar Dupixent.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Dupixent:
Dupixent no es un medicamento de rescatey no se debe usar para tratar un ataque repentino de asma o EPOC.
Cada vez que tenga un nuevo envase de Dupixent, es importante que anote el nombre del medicamento, la fecha de administración y el número de lote (que se encuentra en el envase después de “Lote”) y guarde esta información en un lugar seguro.
Reacciones alérgicas
- De forma rara, Dupixent puede causar efectos adversos graves, incluyendo reacciones alérgicas (hipersensibilidad), reacción anafiláctica y angioedema. Estas reacciones pueden ocurrir desde minutos después de la administración de Dupixent hasta siete días después de la misma. Mientras está utilizando Dupixent debe observar los signos de estas enfermedades (es decir, problemas respiratorios, hinchazón de la cara, labios, boca, garganta o lengua, desmayo, mareo, sensación de mareo (presión arterial baja), fiebre, sensación de malestar general, inflamación de los ganglios linfáticos, habones, picor, dolor en las articulaciones, erupción cutánea). Dichos signos se enumeran en "Efectos adversos graves" en la sección 4.
- Deje de usar Dupixent y dígale a su médico o consiga ayuda médica inmediatamente si nota cualquier signo de una reacción alérgica.
Enfermedades eosinofílicas
- Rara vez, los pacientes que toman un medicamento para el asma pueden presentar una inflamación de los vasos sanguíneos o de los pulmones como resultado del aumento de un cierto tipo de glóbulos blancos (eosinofilia).
- No se sabe si esto es causado por Dupixent. Esto generalmente, pero no siempre, ocurre en personas que también toman un medicamento esteroideo que se ha interrumpido o se está reduciendo su dosis.
- Si presenta una combinación de síntomas que incluye una enfermedad similar a la gripe, hormigueo o entumecimiento de los brazos o piernas, empeoramiento de los síntomas pulmonares y/o erupción, informe a su médico inmediatamente.
Infección parasitaria (parásitos intestinales)
- Dupixent puede debilitar su resistencia a las infecciones causadas por parásitos. Si ya tiene una infección por parásitos, se debe tratar antes de comenzar el tratamiento con Dupixent.
- Consulte con su médico si tiene diarrea, gases, malestar estomacal, heces grasientas y deshidratación que pueden ser un signo de una infección parasitaria.
- Si vive en una región donde estas infecciones son frecuentes o si está viajando a esa región consulte con su médico.
AsmaSi tiene asma y está tomando medicación para el asma, no cambie ni deje de tomar su medicación para el asma sin consultar con su médico. Consulte con su médico antes de interrumpir el tratamiento con Dupixent o si su asma no se controla o empeora durante el tratamiento con este medicamento.
Problemas oculares
Consulte con su médico si aparecen o empeoran los problemas en los ojos, incluyendo dolor en los mismos o cambios en la vista.
Niños y adolescentes
- No se conocen todavía la seguridad y beneficios de Dupixent en niños menores de 6 meses de edad con dermatitis atópica.
- No se conocen todavía la seguridad y beneficios de Dupixent en niños menores de 6 años de edad con asma.
- No se conocen todavía la seguridad y beneficios de Dupixent en niños menores de 18 años de edad con RSCcPN.
- No se conocen la seguridad y beneficios de Dupixent en niños menores de 18 años de edad con PN.
- No se conocen todavía la seguridad y beneficios de Dupixent en niños menores de 1 año de edad, o con un peso corporal < 15 kg con EEo.
- No se conocen la seguridad y beneficios de Dupixent en niños menores de 18 años de edad con EPOC.
Otros medicamentos y Dupixent
Informe a su médico o farmacéutico
- si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- si se ha vacunado recientemente o tiene que vacunarse.
Otros medicamentos para el asma
No interrumpa o reduzca sus medicamentos para el asma, a menos que se lo indique su médico.
- Estos medicamentos (especialmente los denominados corticosteroides) se deben interrumpir gradualmente.
- Esto se debe hacer bajo la supervisión directa de su médico y dependiendo de su respuesta a Dupixent.
Embarazo y lactancia
- Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Se desconocen los efectos de este medicamento en mujeres embarazadas; por lo tanto, es preferible evitar el uso de Dupixent en el embarazo a menos que su médico le aconseje hacerlo.
- Si está en periodo de lactancia o tiene intención de dar el pecho, consulte a su médico antes de utilizar este medicamento. Su médico y usted deben decidir si dará el pecho o utilizará Dupixent. No debe hacer ambas cosas a la vez.
Conducción y uso de máquinas
Es poco probable que Dupixent influya en su capacidad para conducir y utilizar máquinas.
Dupixent contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis de 300 mg; esto es, esencialmente “exento de sodio”.
Dupixent contiene polisorbato
Este medicamento contiene 4 mg de polisorbato 80 en cada dosis de 300 mg (2 ml). Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene o su hijo tiene cualquier alergia conocida.
3. Cómo usar Dupixent
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Qué dosis de Dupixent recibirá
Su médico decidirá qué dosis de Dupixent es adecuada para usted.
Dosis recomendada en adultos con dermatitis atópica
Para pacientes con dermatitis atópica, la dosis recomendada de Dupixent es:
- Una dosis inicial de 600 mg (dos inyecciones de 300 mg)
- Seguida de 300 mg cada dos semanas por inyección subcutánea.
Dosis recomendada en adolescentes con dermatitis atópica
La dosis recomendada de Dupixent para adolescentes (de 12 a 17 años de edad) con dermatitis atópica se basa en el peso corporal:
Peso corporal del paciente | Dosis inicial | Dosis posteriores (cada dos semanas) |
menos de 60 kg | 400 mg (dos inyecciones de 200 mg) | 200 mg |
60 kg o más | 600 mg (dos inyecciones de 300 mg) | 300 mg |
Dosis recomendada en niños de 6 a 11 años de edad con dermatitis atópica
La dosis recomendada de Dupixent para niños (de 6 a 11 años de edad) con dermatitis atópica se basa en el peso corporal:
Peso corporal del paciente | Dosis inicial | Dosis posteriores |
15 kg a menos de 60 kg | 300 mg (una inyección de 300 mg) en el día 1, seguidos de 300 mg en el día 15 | 300 mg cada 4 semanas*, comenzando 4 semanas después de la dosis del día 15 |
60 kg o más | 600 mg (dos inyecciones de 300 mg) | 300 mg cada dos semanas |
- La dosis se puede aumentar a 200 mg cada dos semanas según la opinión del médico.
Dosis recomendada en niños de 6 meses a 5 años de edad con dermatitis atópica
La dosis recomendada de Dupixent para niños de 6 meses a 5 años de edad con dermatitis atópica se basa en el peso corporal:
Peso corporal del paciente | Dosis inicial | Dosis posteriores |
5 kg a menos de 15 kg | 200 mg (una inyección de 200 mg) | 200 mg cada 4 semanas |
15 kg a menos de 30 kg | 300 mg (una inyección de 300 mg) | 300 mg cada 4 semanas |
Dosis recomendada en pacientes adultos y adolescentes con asma (a partir de 12 años de edad)
Para pacientes con asma grave y que toman corticosteroides orales o para pacientes con asma grave y dermatitis atópica comórbida de moderada a grave o adultos con rinosinusitis crónica comórbida grave con poliposis nasal, la dosis recomendada de Dupixent es:
- Una dosis inicial de 600 mg (dos inyecciones de 300 mg)
- Seguida de 300 mg administrados cada dos semanas mediante inyección subcutánea.
Para el resto de pacientes con asma grave, la dosis recomendada de Dupixent es:
- Una dosis inicial de 400 mg (dos inyecciones de 200 mg)
- Seguida de 200 mg administrados cada dos semanas mediante inyección subcutánea.
Dosis recomendada en niños con asma
La dosis recomendada de Dupixent para niños (de 6 a 11 años de edad) con asma se basa en el peso corporal:
Peso corporal del paciente | Dosis inicial y posteriores |
15 kg a menos de 30 kg | 300 mg cada 4 semanas |
30 kg a menos de 60 kg | 200 mg cada dos semanas o 300 mg cada 4 semanas |
60 kg o más | 200 mg cada dos semanas |
Para pacientes de 6 a 11 años con asma y dermatitis atópica grave coexistente, su médico decidirá qué dosis de Dupixent es adecuada para usted.
Dosis recomendada en adultos con rinosinusitis crónica con poliposis nasal (RSCcPN)
En RSCcPN, la primera dosis recomendada de Dupixent es de 300 mg seguida de 300 mg cada dos semanas por inyección subcutánea.
Dosis recomendada en adultos con prurigo nodular (PN)
Para pacientes con prurigo nodular, la dosis recomendada de Dupixent es:
- Una dosis inicial de 600 mg (dos inyecciones de 300 mg)
- Seguida de 300 mg administrados cada dos semanas mediante inyección subcutánea.
Dosis recomendada en adultos, adolescentes y niños (a partir de 1 año de edad) con esofagitis eosinofílica (EEo)
Peso corporal | Dosis |
≥15 kg a <30 kg | 200 mg cada dos semanas |
≥30 kg a <40 kg | 300 mg cada dos semanas |
≥40 kg | 300 mg cada semana |
Dosis recomendada en adultos con enfermedad pulmonar obstructiva crónica (EPOC)
En EPOC, la dosis recomendada de Dupixent es de 300 mg administrados cada dos semanas mediante inyección subcutánea.
Inyección de Dupixent
Dupixent se administra por inyección debajo de su piel (inyección subcutánea). Su médico o enfermero y usted deben decidir si se debe inyectar Dupixent usted mismo.
Antes de inyectarse Dupixent usted mismo, debe haber sido entrenado correctamente por su médico o enfermero. Su inyección de Dupixent también puede ser administrada por un cuidador después de un entrenamiento adecuado por parte de un médico o enfermero.Cada jeringa precargada contiene una dosis de Dupixent (300 mg). No agite la jeringa precargada.Lea cuidadosamente las “Instrucciones de Uso” incluidas al final del prospecto antes de usar Dupixent.
Si usa más Dupixent del que debe
Si usa más Dupixent del que debe o se ha administrado la dosis demasiado pronto, consulte a su médico, farmacéutico o enfermero.
Si olvidó usar Dupixent
Si ha olvidado inyectarse una dosis de Dupixent, consulte a su médico, farmacéutico o enfermero.
Además,
Si su pauta de dosis es cada semanay olvida una dosis de Dupixent:
- administre la inyección de Dupixent tan pronto como sea posible y empiece una nueva pauta de dosis cada semana desde el momento en que se acuerde de administrarse su inyección de Dupixent.
Si su pauta de dosis es cada dos semanasy olvida una dosis de Dupixent:
- administre la inyección de Dupixent dentro de los 7 días siguientes a la dosis olvidada, luego siga con su pauta original.
- si la dosis olvidada no se administra dentro de los 7 días, espere hasta la siguiente dosis programada para administrar su inyección de Dupixent.
Si su pauta de dosis es cada 4 semanasy olvida una dosis de Dupixent:
- administre la inyección de Dupixent dentro de los 7 días siguientes a la dosis olvidada, luego siga con su pauta original.
- si la dosis olvidada no se administra dentro de los 7 días, empiece una nueva pauta de dosis cada 4 semanas desde el momento en que se acuerde de administrarse su inyección de Dupixent.
Si interrumpe el tratamiento con Dupixent
No interrumpa el tratamiento con Dupixent sin comentarlo primero con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Dupixent puede causar efectos adversos graves, incluyendo reacciones alérgicas raras (hipersensibilidad), incluyendo reacción anafiláctica, enfermedad del suero, reacción tipo enfermedad del suero; los signos pueden incluir:
- problemas respiratorios
- hinchazón de la cara, labios, boca, garganta o lengua (angioedema)
- desmayo, mareo, sensación de mareo (presión arterial baja)
- fiebre
- sensación de malestar general
- inflamación de los ganglios linfáticos
- habones
- picor
- dolor en las articulaciones
- erupción cutánea
Si desarrolla una reacción alérgica, deje de usar Dupixent y consulte a su médico de inmediato.
Otros efectos adversos
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- reacciones en el lugar de la inyección (p.ej., enrojecimiento localizado, hinchazón, picor, dolor, hematomas)
- enrojecimiento y picor de ojos
- infección de ojos
- herpes (en los labios y la piel)
- un aumento de cierto número de glóbulos blancos (eosinófilos)
- dolor en las articulaciones (artralgia)
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- hinchazón de la cara, labios, boca, garganta o lengua (angioedema)
- picor, enrojecimiento e hinchazón de párpados
- inflamación de la superficie del ojo, a veces con visión borrosa (queratitis)
- erupción o enrojecimiento facial
- sequedad de ojos
Raras(pueden afectar hasta 1 de cada 1 000 personas):
- reacciones alérgicas graves (hipersensibilidad)
- úlceras en la capa transparente externa del ojo, a veces con visión borrosa (queratitis ulcerosa)
Efectos adversos adicionales en niños de 6 a 11 años con asma
Frecuentes: lombrices (enterobiasis)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Dupixent
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar. Conservar en el envase original para protegerlo de la luz.
Si es necesario, la jeringa precargada se puede sacar de la nevera y conservar en el envase durante un máximo de 14 días a temperatura ambiente hasta 25 °C, protegida de la luz. La fecha en que se saca de la nevera se anotará en el espacio provisto para ello en el envase exterior. El envase se debe desechar si se deja fuera de la nevera durante más de 14 días o si ha pasado la fecha de caducidad.
No utilice este medicamento si observa que el medicamento está turbio, decolorado o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su médico, farmacéutico o enfermero cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Dupixent
- El principio activo es dupilumab.
- Cada jeringa precargada contiene 300 mg de dupilumab en 2 ml de solución inyectable (inyectable).
- Los demás componentes son L-Arginina monohidrocloruro, L-Histidina, L-Histidina monohidrocloruro monohidrato, polisorbato 80 (E 433), acetato de sodio trihidrato, ácido acético glacial (E 260), sacarosa y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Dupixent es una solución transparente a ligeramente opalescente, de incolora a amarillo pálido que se presenta en una jeringa precargada de vidrio con protector de aguja.
Dupixent está disponible como jeringas precargadas de 300 mg en un envase conteniendo 1 o 2 jeringas precargadas o en un envase múltiple conteniendo 6 (3 envases de 2) jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
Francia
Responsable de la fabricación
SANOFI WINTHROP INDUSTRIE
1051 Boulevard Industriel,
76580 LE TRAIT,
FRANCIA
Sanofi-Aventis Deutschland GmbH
Brüningstrasse 50
Industriepark Hoechst
65926 FRANKFURT AM MAIN
ALEMANIA
Genzyme Ireland Limited
IDA Industrial Park
Old Kilmeaden Road
Waterford
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Ceská republika Sanofi, s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. aus dem Ausland: +49 69 305 70 13 | Nederland Sanofi B.V. Tel: + 31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Ελλáδα Sanofi-Aventis Μονοπρóσωπη AEBE Τηλ: +30 210 900 16 00 | Österreich sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda Tel: +351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536389 | Suomi/Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Κúπρος C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom(Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
Dupixent 300mg solución inyectable en jeringa precargada con protector de aguja
dupilumab
Instrucciones de uso
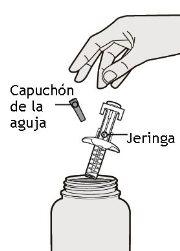
En este dibujo se muestran las partes de la jeringa precargada de Dupixent con protector de aguja.
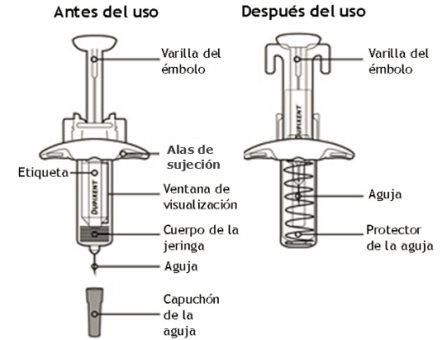
Información importante
Este dispositivo es una jeringa precargada de un solo uso. Contiene 300 mg de Dupixent para inyección debajo de la piel (inyección subcutánea).
No intente administrarse la inyección a sí mismo o a otra persona, a menos que haya recibido formación por parte de su profesional sanitario. En adolescentes a partir de 12 años de edad, se recomienda que Dupixent se administre por o bajo la supervisión de un adulto. En niños menores de 12 años de edad, Dupixent se debe administrar por un cuidador.
- Lea todas las instrucciones detenidamente antes de utilizar la jeringa.
- Compruebe con su profesional sanitario con qué frecuencia se debe inyectar el medicamento.
- Consulte a su profesional sanitario para que le muestre cómo usar correctamente la jeringa antes de usarla por primera vez.
- Cambie el lugar de la inyección cada vez que se la ponga.
- Nouse la jeringa si se ha caído sobre una superficie dura o si ha sufrido daños.
- Nouse la jeringa si falta el capuchón de la aguja o este no está debidamente sujeto.
- Notoque la varilla del émbolo hasta que no esté preparado para la inyección.
- Noinyecte a través de la ropa.
- Notrate de eliminar las burbujas de aire en la jeringa.
- Para ayudar a prevenir lesiones accidentales a causa de la aguja, cada jeringa precargada viene con un protector de aguja que se activa automáticamente para cubrir la aguja después de haber administrado la inyección.
- Nuncatire hacia atrás de la varilla del émbolo.
- Noreutilice la jeringa.
Conservación de Dupixent
- Mantenga la(s) jeringa(s) fuera del alcance de los niños.
- Mantenga las jeringas sin usar en el envase original y consérvelas en la nevera entre 2 °C y 8 °C.
- Nomantenga Dupixent a temperatura ambiente (< 25 °C) durante más de 14 días. Si necesita sacar el envase de la nevera de forma permanente, escriba la fecha en que lo saca en el espacio provisto para ello en el envase exterior, y use Dupixent en los 14 días siguientes.
- Noagite la jeringa en ningún momento.
- Nocaliente la jeringa.
- Nocongele la jeringa.
- Noexponga la jeringa a la luz solar directa.
Paso 1: Sacar
Saque la jeringa del envase cogiéndola por el medio del cuerpo de la jeringa.
 No quite el capuchón de la aguja hasta que usted no esté preparado para la inyección.
No quite el capuchón de la aguja hasta que usted no esté preparado para la inyección.
 No use la jeringa si esta se ha caído sobre una superficie dura o si ha sufrido daños.
No use la jeringa si esta se ha caído sobre una superficie dura o si ha sufrido daños.
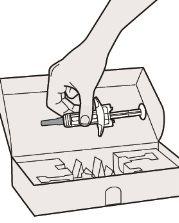
Paso 2: Preparar
Asegúrese que tiene lo siguiente:
- la jeringa precargada de Dupixent
- 1 toallita con alcohol*
- 1 bola de algodón o gasa*
- un contenedor para objetos punzantes* (ver el Paso 12)
- Elementos no incluidos en el envase
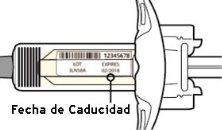
Mire la etiqueta:
- Compruebe la fecha de caducidad.
- Compruebe que tiene el medicamento y la dosis correctos.
 No use la jeringa si la fecha de caducidad ya ha pasado.
No use la jeringa si la fecha de caducidad ya ha pasado.
 No guarde Dupixent a temperatura ambiente durante más de 14 días.
No guarde Dupixent a temperatura ambiente durante más de 14 días.
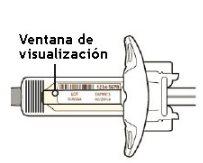
Paso 3: Comprobar
Mire el medicamento a través de la ventana de visualización de la jeringa:
Compruebe que el líquido es transparente e incoloro a color amarillo pálido.
Nota: es posible que vea una burbuja de aire, pero es normal.
 No use la jeringa si el líquido está turbio o decolorado, o si contiene copos o partículas.
No use la jeringa si el líquido está turbio o decolorado, o si contiene copos o partículas.
Paso 4: Esperar 45minutos
Coloque la jeringa sobre una superficie plana durante al menos 45 minutos y deje que alcance la temperatura ambiente de forma natural.
 No caliente la jeringa en un microondas, agua caliente o luz solar directa.
No caliente la jeringa en un microondas, agua caliente o luz solar directa.
 No exponga la jeringa a la luz solar directa.
No exponga la jeringa a la luz solar directa.
 No mantenga Dupixent a temperatura ambiente durante más de 14 días.
No mantenga Dupixent a temperatura ambiente durante más de 14 días.
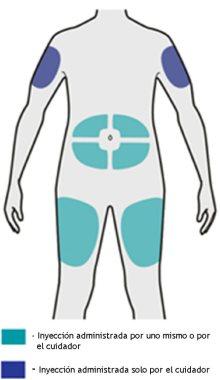
Paso 5: Elegir
Seleccione el lugar de la inyección.
- Puede inyectar el medicamento en su muslo o vientre (abdomen), evitando el área de unos 5 cm alrededor de su ombligo.
- Si alguien le pone la inyección, también puede hacerlo en la parte superior del brazo.
- Cambie el lugar de la inyección cada vez que se la ponga.
 No realice la inyección en la piel sensible, dañada ni con hematomas o cicatrices.
No realice la inyección en la piel sensible, dañada ni con hematomas o cicatrices.
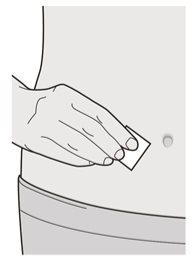
Paso 6: Limpiar
Lávese las manos.
Desinfecte el lugar de la inyección con una toallita con alcohol.
Deje que la piel se seque antes de proceder con la inyección.
 No vuelva a tocar el lugar de la inyección ni sople encima antes de la inyección.
No vuelva a tocar el lugar de la inyección ni sople encima antes de la inyección.

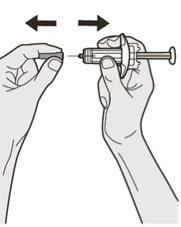
Paso 7: Tirar
Sujete la jeringa por el medio del cuerpo de la jeringa apuntando la aguja en dirección contraria a usted y retire el capuchón de la aguja.
 No vuelva a poner el capuchón en la aguja.
No vuelva a poner el capuchón en la aguja.
 No toque la aguja.
No toque la aguja.
Inyecte el medicamento inmediatamente después de quitar el capuchón de la aguja.
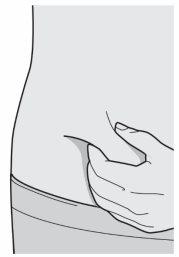
Paso 8: Pellizcar
Pellizque un pliegue de piel en el lugar de la inyección, como se muestra en el dibujo.
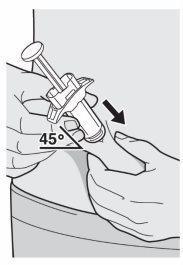
Paso 9: Introducir
Introduzca la aguja por completo en el pliegue de piel en un ángulo de aproximadamente 45°.
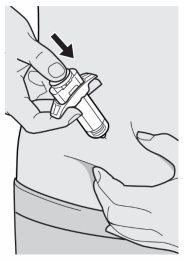
Paso 10: Empujar
Relaje el pellizco.
Empuje la varilla del émbolo hacia abajo lentamente y de forma ininterrumpida hasta que se detenga y la jeringa esté vacía.
Nota: notará un poco de resistencia, lo cual es normal.
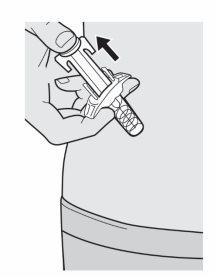
Paso11: Soltar y Retirar
Levante el pulgar para soltar la varilla del émbolo hasta que la aguja quede cubierta por el protector de la aguja y luego retire la jeringa del lugar de la inyección.
Si ve algo de sangre, presione suavemente en el lugar de la inyección con una bola de algodón o gasa.
 No vuelva a poner el capuchón en la aguja.
No vuelva a poner el capuchón en la aguja.
 No se frote la piel después de ponerse la inyección.
No se frote la piel después de ponerse la inyección.
Paso 12: Desechar
Tire la jeringa y el capuchón de la aguja en un contenedor para objetos punzantes.
 No vuelva a poner el capuchón en la aguja.
No vuelva a poner el capuchón en la aguja.
Mantenga siempre el contenedor fuera del alcance de los niños.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DUPIXENT 300 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: DupilumabFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: DupilumabFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: DupilumabFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para DUPIXENT 300 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DUPIXENT 300 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes









