
CYRAMZA 10 MG/ML CONCENTRADO PARA SOLUCION PARA PERFUSION
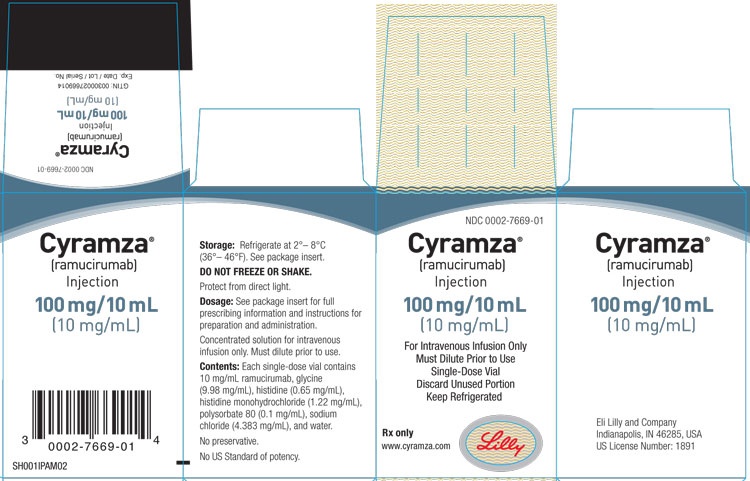

Cómo usar CYRAMZA 10 MG/ML CONCENTRADO PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Cyramza 10 mg/ml concentrado para solución para perfusión
ramucirumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Cyramza y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cyramza
- Cómo usar Cyramza
- Posibles efectos adversos
- Conservación de Cyramza
- Contenido del envase e información adicional
1. Qué es Cyramza y para qué se utiliza
Cyramza es un medicamento para el cáncer que contiene ramucirumab como principio activo, el cual es un anticuerpo monoclonal. Es una proteína especializada que puede reconocer y unirse a otra proteína que se encuentra en los vasos sanguíneos llamada “Receptor de VEGF tipo 2”. Este receptor es necesario en el desarrollo de nuevos vasos sanguíneos. Para crecer, el cáncer necesita desarrollar nuevos vasos sanguíneos. Al unirse y bloquear al “Receptor de VEGF tipo 2”, el medicamento interrumpe el aporte sanguíneo a las células cancerígenas.
Cyramza también se utiliza en combinación con paclitaxel, otro medicamento anticanceroso, para el tratamiento del cáncer de estómago avanzado (o cáncer de la unión entre el esófago y el estómago) en adultos cuya enfermedad ha empeorado tras el tratamiento con medicamentos para tratar el cáncer.
Cyramza se utiliza para el tratamiento del cáncer de estómago avanzado (o cáncer de la unión entre el esófago y el estómago) en adultos cuya enfermedad ha empeorado después de tratamiento con medicamentos para tratar el cáncer y para quienes el tratamiento de Cyramza en combinación con paclitaxel no sea adecuado.
Cyramza se utiliza para el tratamiento de cáncer de colon o recto (partes del intestino grueso) en adultos. Se administra con otro medicamento llamado “FOLFIRI”, que incluye “5-fluorouracilo”, “ácido folínico” e “irinotecán”.
Cyramza se administra en combinación con erlotinib, otro medicamento anticanceroso, como terapia en primera línea para el tratamiento de pacientes adultos con cáncer de pulmón de células no pequeñas en estado avanzado cuando las células tumorales presentan unos cambios específicos (mutaciones) en el gen del receptor de factor de crecimiento epidérmico.
Cyramza se administra en combinación con docetaxel, otro medicamento anticanceroso, para el tratamiento de pacientes adultos con cáncer de pulmón en estado avanzado cuya enfermedad ha empeorado tras el tratamiento con medicamentos para tratar el cáncer.
Cyramza se utiliza para tratar el cáncer de hígado que está avanzado o que no se puede extirpar mediante cirugía, en adultos que han sido tratados previamente con otro medicamento contra el cáncer (sorafenib) y que tienen un nivel elevado de una proteína específica en la sangre (alfa fetoproteína).
2. Qué necesita saber antes de empezar a usar Cyramza
No use Cyramza:
- si es alérgico a ramucirumab o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si los rayos-X muestran signos de que el cáncer de pulmón tiene una cavidad o agujero o si el cáncer de pulmón se encuentra pegado a vasos sanguíneos mayores.
Advertencias y precauciones
Consulte a su médico o enfermero antesde empezar a usar Cyramza si:
- tiene una enfermedad que aumenta el riesgo de hemorragia. También consulte a su médico si está tomando otros medicamentos que aumentan el riesgo de hemorragia o que afectan a la capacidad de coagulación de la sangre. En estos casos, su médico llevará a cabo análisis de sangre de forma regular para controlar el riesgo de hemorragia.
- tiene cáncer de hígado y ha tenido un sangrado previo por varices en su tubo digestivo (esófago) o tiene hipertensión de la vena porta, la cual lleva la sangre desde el intestino y el bazo al hígado.
- tiene cáncer de pulmón y ha tenido un sangrado en el pulmón recientemente (tos con sangre roja brillante) o está tomando de forma continua medicamentos antiinflamatorios no esteroideos o medicamentos que afectan a la capacidad de coagulación de la sangre.
- tiene la tensión sanguínea alta. Cyramza puede aumentar la aparición de tensión sanguínea alta. Su médico se asegurará de que si ya presenta una tensión sanguínea alta ésta tiene que estar bajo control antes de empezar el tratamiento con Cyramza. Durante el tratamiento con Cyramza, su médico controlará su tensión sanguínea y ajustará la medicación antihipertensiva si fuese necesario. Se puede interrumpir temporalmente el tratamiento con Cyramza hasta que la tensión sanguínea alta esté controlada con medicamentos o se puede interrumpir de forma permanente si no se puede controlar adecuadamente.
- tiene o ha tenido un aneurisma (aumento y debilitamiento de la pared de un vaso sanguíneo) o un desgarro en la pared de un vaso sanguíneo.
- va a ser operado, si usted ha sido operado recientemente o si tras la operación su herida no se está curando bien. Cyramza puede aumentar el riesgo de problemas con la curación de heridas. No debe recibir Cyramza durante al menos 4 semanas antes de que se lleve a cabo la operación programada y su médico decidirá cuando reanudar el tratamiento. Si durante el tratamiento tiene una herida que cura mal, la dosis de Cyramza se interrumpirá hasta que la herida esté completamente curada.
- tiene una enfermedad del hígado grave (“cirrosis”) y enfermedades asociadas tales como acumulación excesiva de fluidos en su abdomen (“ascitis”). Su médico le comentará si los beneficios potenciales del tratamiento justifican el aumento de posibles riesgos para usted. Si tiene cáncer de hígado su médico le controlará los signos y síntomas de confusión y/o desorientación asociados con problemas de hígado crónicos y suspenderá el tratamiento con Cyramza si desarrolla esos signos y síntomas.
- tiene problemas de riñón graves. Los datos disponibles sobre el uso de Cyramza en pacientes con trastorno grave de la función renal son limitados.
Consulte con su médico o enfermero inmediatamentesi alguna de las siguientes afirmaciones le afectan (o no está seguro) durante el tratamientocon Cyramza o en cualquier otro momento después:
- Bloqueo de las arterias por un trombo(“enfermedad tromboembólica arterial”):
Cyramza puede provocar un trombo en sus arterias. Los trombos en las arterias pueden causar enfermedades graves, incluyendo infarto cardiaco o embolia cerebral. Los síntomas del infarto cardiaco pueden incluir dolor u opresión en el pecho. Los síntomas de una embolia cerebral pueden incluir entumecimiento repentino o debilidad del brazo, piernas o cara, confusión, dificultad para hablar o entender a los demás, dificultad repentina al caminar o pérdida de equilibrio o descoordinación o mareo repentino. Si desarrolla un trombo en sus arterias, el tratamiento con Cyramza se interrumpirá de forma permanente.
- Perforación en la pared del intestino(“perforación gastrointestinal”): Cyramza puede aumentar el riesgo de que se desarrollen perforaciones en la pared de su intestino. Los síntomas incluyen dolor abdominal grave, vómitos, fiebre o escalofríos. Si se desarrolla una perforación en la pared del intestino, el tratamiento con Cyramza se interrumpirá de forma permanente.
- Hemorragia grave:Cyramza puede aumentar el riesgo de sangrado grave. Los síntomas pueden
incluir: cansancio extremo, debilidad, mareo o cambios en el color de sus heces. Si sufre hemorragia grave, el tratamiento con Cyramza se interrumpirá de forma permanente.
- Reacciones relacionadas con la perfusión:pueden ocurrir reacciones relacionadas con la perfusión durante el tratamiento, debido a que Cyramza se administra mediante perfusión intravenosa por goteo (ver sección 3). Su médico o enfermero controlorá la aparición de efectos adversos durante la perfusión. Los síntomas pueden incluir: aumento de la tensión muscular, dolor de espalda, dolor y/u opresión en el pecho, escalofríos, enrojecimiento, dificultad para respirar, silbidos al respirar y sensación de hormigueo o entumecimiento en las manos o los pies. En casos graves, los síntomas pueden incluir dificultad respiratoria producida por un estrechamiento de las vías respiratorias, pulso acelerado y sensación de desmayo. Si sufre una reacción grave relacionada con la perfusión, el tratamiento con Cyramza se interrumpirá de forma permanente.
- Una enfermedad cerebral rara pero gravellamada "síndrome de encefalopatía posterior reversible" o "PRES" (por sus siglas en inglés): Cyramza puede aumentar el riesgo de desarrollar esta enfermedad cerebral. Los síntomas pueden incluir ataques (convulsiones), dolor de cabeza, sentirse enfermo (náuseas), vómitos, ceguera o disminución del nivel de conciencia, con o sin presión arterial alta. Interrumpa el tratamiento de Cyramza si experimenta esta enfermedad cerebral.
- Insuficiencia cardiaca: Cyramza, cuando se administra en combinación con quimioterapia o erlotinib, puede aumentar el riesgo de insuficiencia cardiaca. Los síntomas pueden incluir debilidad y cansancio, hinchazón y acumulación de líquido en los pulmones, lo que puede causar dificultad para respirar. Se evaluarán sus síntomas y se podrá considerar la suspensión de su tratamiento con Cyramza.
- Conductos anómalos en el interior del cuerpo(“fístula”): Cyramza puede aumentar el riesgo de aparición de conductos anómalos en el interior del cuerpo entre órganos internos y la piel u otros tejidos. Si desarrolla una fístula, el tratamiento con Cyramza se interrumpirá de forma permanente.
- Prueba de orina anormal(“proteinuria”): Cyramza puede aumentar el riesgo de desarrollar o empeorar los niveles anormales de proteína en orina. El tratamiento con Cyramza puede necesitar ser interrumpido temporalmente hasta que los niveles de proteína en orina desciendan y entonces ser reanudado a dosis más bajas, o interrumpirse de forma permanente si los niveles de proteínas en orina no se reducen lo suficiente.
- Inflamación de la boca(“estomatitis”): Cyramza, cuando se administra en combinación con quimioterapia puede aumentar el riesgo de desarrollar inflamación de la boca. Los síntomas pueden incluir sensación de quemazón en la boca, úlceras, ampollas o inflamación. Su médico puede recetarle un tratamiento para ayudarle con los síntomas.
- Fiebre o infección:Durante el tratamiento puede tener una temperatura de 38 ºC o más (dado que puede tener una cantidad menor de lo normal de células blancas de la sangre lo cual es muy común). Los síntomas pueden incluir sudoración u otros signos de infección, tales como dolor de cabeza, dolor en las extremidades o disminución del apetito. La infección (sepsis) podría ser grave y llevar a la muerte.
- Pacientes de edad avanzada con cáncer de pulmón:Su médico considerará cuidadosamente el tratamiento más apropiado para usted.
Niños y adolescentes
Cyramza no se debe administrar a pacientes menores de 18 años debido a que no hay información sobre cómo funciona en este grupo de edad.
Uso de Cyramza con otros medicamentos
Informe a su médico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento. Esto incluye medicamentos obtenidos sin receta y a base de plantas.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Debe evitar quedarse embarazada mientras recibe este medicamento y durante al menos 3 meses tras recibir la última dosis de Cyramza. Consulte a su médico sobre el mejor método anticonceptivo para usted.
Como Cyramza impide el desarrollo de nuevos vasos sanguíneos, puede disminuir la probabilidad de quedarse embarazada o mantener un embarazo. Puede también dañar al feto. No debe usar este medicamento durante el embarazo. Si llega a quedarse embarazada durante el tratamiento con Cyramza, su médico comentará con usted si los beneficios del tratamiento son mayores que cualquier posible riesgo para usted o su bebé.
No se conoce si el medicamento pasa a la leche materna y podría afectar al lactante. Por lo tanto, no debe dar el pecho a su bebé durante el tratamiento con Cyramza y durante al menos 3 meses tras recibir la última dosis.
Conducción y uso de máquinas
La influencia de Cyramza sobre la capacidad para conducir y utilizar máquinas es nula o insignificante. Si usted presenta cualquier síntoma que afecte a su habilidad para concentrarse y reaccionar, no conduzca ni use máquinas hasta que los síntomas desaparezcan.
Cyramza contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) en cada vial de 10 ml; esto es, esencialmente “exento de sodio”.
Este medicamento contiene aproximadamente 85 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada vial de 50 ml. Esto equivale aproximadamente al 4% de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo usar Cyramza
Este tratamiento contra el cáncer le será administrado por su médico o enfermero.
Dosis y frecuencia de administración
La cantidad adecuada de Cyramza necesaria para tratar su enfermedad será calculada por su médico o farmacéutico hospitalario dependiendo de su peso corporal.
La dosis recomendada de Cyramza para el tratamiento del cáncer gástrico, para el tratamiento de cáncer avanzado de colon o recto y para el tratamiento de cáncer de hígado es 8 mg por kilo de peso una vez cada 2 semanas.
La dosis recomendada de Cyramza para el tratamiento de cáncer de pulmón es de 10 mg por kilo de peso una vez cada 2 semanas cuando se administra en combinación con erlotinib o una vez cada 3 semanas cuando se administra en combinación con docetaxel.
El número de perfusiones que recibirá dependerá de cómo esté respondiendo al tratamiento. Su médico lo comentará con usted.
Medicación previa
Puede recibir otro medicamento para reducir el riesgo de reacciones relacionadas con la perfusión antes de recibir Cyramza. Si presenta una reacción relacionada con la perfusión durante el tratamiento con Cyramza, debe recibir medicación previa en todas las perfusiones posteriores.
Ajuste de dosis
Durante cada perfusión, su médico o enfermero comprobarán si presenta efectos adversos.
Si presenta una reacción relacionada con la perfusión durante el tratamiento, la duración final de la perfusión aumentará para el resto de esa perfusión y para las siguientes.
La cantidad de proteínas en la orina será comprobada regularmente durante el tratamiento. Cyramza se puede interrumpir de forma temporal según la medida del nivel de proteínas. Una vez que los niveles de proteína en orina han disminuido hasta ciertos niveles, el tratamiento se puede reanudar a una dosis menor.
Vía y forma de administración
Cyramza es un concentrado para solución para perfusión (también llamado “concentración estéril”). Un farmacéutico hospitalario, enfermero o médico diluirán el contenido del vial en una solución de cloruro de sodio 9 mg/ml (0,9%) antes de usarlo. Este medicamento se administra mediante perfusión por goteo durante aproximadamente 60 minutos.
El tratamiento con Cyramza se interrumpirá de forma temporal si:
- presenta tensión sanguínea alta, hasta que se controle con antihipertensivos
- presenta problemas para la curación de heridas, hasta que la herida se cure
- tiene una cirugía programada, 4 semanas antes de la misma
El tratamiento con Cyramza se interrumpirá de forma permanente si:
- presenta un trombo en sus arterias
- presenta una perforación en la pared del intestino
- experimenta hemorragia grave
- experimenta una reacción grave relacionada con la perfusión
- presenta tensión sanguínea alta que no se puede controlar con medicamentos
- está pasando a su orina más cantidad de proteínas de lo normal o presenta una enfermedad grave en los riñones (síndrome nefrótico)
- presenta un conducto anómalo en el interior de su cuerpo entre órganos internos y la piel u otros tejidos (fístula)
- desarrolla confusión y/o desorientación asociados con problemas de hígado crónicos
- disminución de la función renal (en el contexto de insuficiencia hepática)
Cuando reciba Cyramza en combinación con paclitaxel o docetaxel
Paclitaxel y también docetaxel se administran por goteo en vena (perfusión intravenosa) durante un periodo de aproximadamente 60 minutos. Si recibe Cyramza en combinación con paclitaxel o docetaxel el mismo día,Cyramza se le administrará primero.
La cantidad de paclitaxel o docetaxel necesaria depende de su superficie corporal. Su médico o farmacéutico hospitalario calculará su superficie corporal midiendo su talla y peso y calculará la dosis adecuada para usted.
La dosis recomendada de paclitaxel es de 80 mg por metro cuadrado (m2) de su superficie corporal una vez a la semana durante 3 semanas seguido de 1 semana sin tratamiento.
La dosis recomendada de docetaxel es 75 mg por metro cuadrado (m2) de su superficie corporal una vez cada 3 semanas. Si es de Asia Oriental, puede recibir una dosis de inicio de docetaxel reducida de 60 mg por m2 de su superficie corporal una vez cada 3 semanas.
Antes de comenzar con cualquier perfusión con paclitaxel, se realizará una analítica para comprobar que el recuento de sus células sanguíneas es suficientemente alto y que su hígado funciona correctamente.
Lea el prospecto de paclitaxel o docetaxel para más información.
Cuando reciba Cyramza en combinación con FOLFIRI
La quimioterapia con FOLFIRI se administra por perfusión intravenosa una vez finalizada la perfusión de Cyramza. Por favor, lea el prospecto del resto de medicamentos que son parte de su tratamiento para comprobar si son apropiados para usted. Si no está seguro, consulte a su médico, farmacéutico o enfermero si hay alguna razón por la que usted no puede utilizar estos medicamentos.
Cuando reciba Cyramza en combinación con erlotinib
Por favor, lea el prospecto de erlotinib para más información sobre erlotinib y comprobar si es apropiado para usted. Si no está seguro, pregunte a su médico, farmacéutico o enfermero si hay alguna razón por la que usted no puede tomar erlotinib.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Consulte con su médico inmediatamentesi sufre cualquiera de los siguientes efectos adversos graves que han sido observados durante el tratamiento con Cyramza (ver también Qué necesita saber antes de empezara usar Cyramza):
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- perforaciones en la pared del intestino:Perforación que se desarrolla en el estómago o intestino. Los síntomas incluyen dolor abdominal grave, vómitos, fiebre o escalofríos.
- hemorragia grave en el intestino:los síntomas pueden incluir cansancio extremo, debilidad, mareo o cambios en el color de sus heces.
- trombos en arterias:los trombos en las arterias puede causar un infarto cardiaco o embolia cerebral. Los síntomas de un infarto cardiaco pueden incluir dolor u opresión en el pecho. Los síntomas de una embolia cerebral pueden incluir entumecimiento repentino o debilidad del brazo, piernas o cara, confusión, dificultad para hablar o entender a los demás, dificultad repentina al caminar o pérdida de equilibrio o descoordinación o mareo repentino.
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas):
- una enfermedad cerebralllamada síndrome de encefalopatía posterior reversible: los síntomas pueden incluir ataques (convulsiones), dolor de cabeza, sentirse enfermo (náuseas), vómitos, ceguera o disminución del nivel de conciencia, con o sin presión arterial alta.
Consulte a su médico si experimenta alguno de los siguientes efectos adversos:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- cansancio o debilidad
- recuento bajo de células blancas en la sangre (puede aumentar el riesgo de infección)
- infecciones
- diarrea
- caída del cabello
- hemorragia nasal
- inflamación del interior de la boca
- tensión sanguínea alta
- reducción del número de glóbulos rojos que puede hacer palidecer la piel
- hinchazón de manos, pies y piernas debido a retención de líquidos
- recuento bajo de plaquetas (células sanguíneas que ayudan a que la sangre coagule)
- dolor abdominal
- proteínas en la orina (prueba de orina anómala)
- dolor de cabeza
- inflamación de las membranas mucosas, tales como los tractos digestivo y respiratorio
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas):
- fiebre acompañada de recuento bajo de células blancas sanguíneas
- niveles bajos en sangre de una proteína llamada albúmina
- reacciones relacionadas con el lugar de la perfusión
- sarpullido
- eritema, inflamación, entumecimiento/hormigueo o dolor y/o descamación de la piel de las manos y/o los pies (llamado síndrome mano-pie)
- ronquera
- sangrado en sus pulmones
- niveles bajos de sodio en sangre (hiponatremia) que puede causar cansancio y confusión o espasmos musculares
- sangrado de las encías
- confusión y/o desorientación en pacientes con problemas de hígado crónicos
- obstrucción intestinal; los síntomas pueden incluir estreñimiento y dolor abdominal
- glándula tiroidea poco activa que puede causar cansancio o aumento de peso (hipotiroidismo)
- crecimiento anormal de vasos sanguíneos
- infecciones graves (sepsis)
- niveles bajos de potasio en sangre (hipopotasemia) que puede causar debilidad muscular, espasmos o ritmo cardiaco anormal
Efectos adversos pocofrecuentes(pueden afectar hasta 1 de cada 100 personas):
- afección cardiaca en la que el músculo cardiaco no bombea la sangre tan bien como debería, lo que provoca dificultad para respirar e hinchazón de las piernas y los pies
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas):
- coagulación sanguínea anormal en pequeños vasos sanguíneos
Frecuencia no conocida(la frecuencia no puede estimarse a partir de los datos disponibles):
- aumento y debilitamiento de la pared de un vaso sanguíneo o desgarro de la pared de un vaso sanguíneo (aneurismas y disecciones arteriales)
Cyramza puede producir cambios en las pruebas de laboratorio. Estos cambios pueden ser, de entre los efectos adversos mencionados anteriormente: recuento bajo de células blancas en sangre, recuento bajo de plaquetas en sangre, niveles bajos de albúmina, potasio o sodio en sangre, presencia de proteínas en la orina.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cyramza
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el cartonaje y la etiqueta del vial después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2°C y 8°C).
No congelar.
Conservar el vial en el embalaje exterior para protegerlo de la luz.
No congelar ni agitar la solución para perfusión. No administrar la solución si nota cualquier partícula o un color extraño.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cyramza
- El principio activo es ramucirumab. Un ml de concentrado para solución para perfusión contiene 10 mg de ramucirumab.
- Cada vial de 10 ml contiene 100 mg de ramucirumab.
- Cada vial de 50 ml contiene 500 mg de ramucirumab.
- Los demás componentes son histidina, monihidrocloruro de histidina, cloruro de sodio, glicina (E640), polisorbato 80 (E433) y agua para preparaciones inyectables (ver en la sección 2 “Cyramza contiene sodio”).
Aspecto del producto y contenido del envase
El concentrado para solución para perfusión (o concentrado estéril) es una solución de aspecto transparente a ligeramente opalescente y de tinte incoloro a ligeramente amarillo que se presenta en un vial de vidrio con un tapón de goma.
Cyramza está disponible en envases de:
- 1 vial de 10 ml
- 2 viales de 10 ml
- 1 vial de 50 ml
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Eli Lilly Nederland B.V.
Papendorpseweg 83
3528 BJ Utrecht
Países Bajos
Responsable de la fabricación
Lilly, S.A.
Avda de la Industria, 30
Alcobendas
28108 Madrid
España
Lilly France Fegersheim
2 rue du Colonel Lilly
67640 Fegersheim
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Belgique/België/BelgienLietuva
Eli Lilly Benelux S.A./N.V. Eli Lilly Lietuva
Tél/Tel: + 32-(0)2 548 84 84 Tel. +370 (5) 2649600
????????Luxembourg/Luxemburg
?? "??? ???? ?????????" ?.?. - ???????? Eli Lilly Benelux S.A./N.V.
???. + 359 2 491 41 40 Tél/Tel: + 32-(0)2 548 84 84
Ceská republikaMagyarország
ELI LILLY CR, s.r.o. Lilly Hungária Kft.
Tel: + 420 234 664 111 Tel: + 36 1 328 5100
DanmarkMalta
Eli Lilly Danmark A/S Charles de Giorgio Ltd.
Tlf: +45 45 26 60 00 Tel: + 356 25600 500
DeutschlandNederland
Lilly Deutschland GmbH Eli Lilly Nederland B.V.
Tel. + 49-(0) 6172 273 2222 Tel: + 31-(0) 30 60 25 800
EestiNorge
Eli Lilly Nederland B.V. Eli Lilly Norge A.S.
Tel: +372 6 817 280 Tlf: + 47 22 88 18 00
Ελλ?δαÖsterreich
ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Eli Lilly Ges.m.b.H.
Τηλ: +30 210 629 4600 Tel: + 43-(0) 1 711 780
EspañaPolska
Lilly S.A. Eli Lilly Polska Sp. z o.o.
Tel: + 34-91 663 50 00 Tel: +48 22 440 33 00
FrancePortugal
Lilly France SAS Lilly Portugal Produtos Farmacêuticos, Lda
Tél: +33-(0) 1 55 49 34 34 Tel: + 351-21-4126600
HrvatskaRomânia
Eli Lilly Hrvatska d.o.o. Eli Lilly România S.R.L.
Tel: +385 1 2350 999 Tel: + 40 21 4023000
IrelandSlovenija
Eli Lilly and Company (Ireland) Limited Eli Lilly farmacevtska družba, d.o.o.
Tel: + 353-(0) 1 661 4377 Tel: +386 (0)1 580 00 10
ÍslandSlovenská republika
Icepharma hf. Eli Lilly Slovakia s.r.o.
Sími + 354 540 8000 Tel: + 421 220 663 111
ItaliaSuomi/Finland
Eli Lilly Italia S.p.A. Oy Eli Lilly Finland Ab
Tel: + 39- 055 42571 Puh/Tel: + 358-(0) 9 85 45 250
Κ?προςSverige
Phadisco Ltd Eli Lilly Sweden AB
Τηλ: +357 22 715000 Tel: + 46-(0) 8 7378800
LatvijaUnited Kingdom
Eli Lilly (Suisse) S.A Parstavnieciba Latvija Eli Lilly and Company Limited
Tel: +371 67364000 Tel: + 44-(0) 1256 315000
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
No agitar el vial.
Preparar la solución para perfusión usando técnicas asépticas para garantizar la esterilidad de la disolución preparada.
Cada vial es de un solo uso. Antes de la dilución se debe comprobar el contenido de los viales para detectar la posible existencia de partículas o decoloración (el concentrado para solución para perfusión debe ser de transparente a ligeramente opalescente y de tinte incoloro a ligeramente amarillo sin partículas visibles). Si se identifican partículas o alteraciones del color, el vial se debe descartar.
Calcular la dosis y el volumen de ramucirumab necesarios para preparar la solución para perfusión. Los viales contienen 100 mg o 500 mg en solución a 10 mg/ml de ramucirumab. Utilizar únicamente como diluyente cloruro de sodio 9 mg/ml (0,9 %) solución inyectable.
En caso de uso de un envase precargado para perfusión intravenosa
Según el volumen de ramucirumab calculado, retirar el volumen correspondiente de solución inyectable de cloruro de sodio 9 mg/ml (0,9 %) del envase precargado de 250 ml para perfusión intravenosa.
El paso del volumen de ramucirumab calculado al envase para perfusión intravenosa se debe realizar asépticamente. El volumen total final del envase debe ser 250 ml. El envase se debe invertir cuidadosamente para garantizar una mezcla adecuada. NO CONGELAR NI AGITAR la solución para perfusión. NO diluir con otras soluciones o coperfundir con otros medicamentos o electrolitos.
En caso de uso de un envase vacío para perfusión intravenosa
El paso de volumen de ramucirumab calculado al envase para perfusión intravenosa vacío se debe realizar asépticamente. Añadir una cantidad suficiente de solución inyectable de cloruro de sodio 9 mg/ml (0,9 %) al envase para alcanzar un volumen total de 250 ml. El envase se debe invertir cuidadosamente para garantizar un mezcla adecuada. NO CONGELAR NI AGITAR. NO diluir con otras soluciones o coperfundir con otros medicamentos o electrolitos.
Tras su dilución y preparación, el medicamento se debe utilizar inmediatamente. Si no lo utiliza inmediatamente, el tiempo y las condiciones de almacenamiento previas al uso son responsabilidad del usuario y normalmente no deberían ser superiores a 24 horas entre 2ºC y 8ºC.
Los medicamentos de administración parenteral, se deben examinar visualmente antes de la administración para descartar la presencia de partículas. Si se identifican partículas, el vial se debe descartar.
Desechar cualquier porción de ramucirumab remanente en el vial dado que el medicamento no contiene conservantes antimicrobianos.
Administrar a través de una bomba de perfusión. Se debe utilizar una vía de perfusión separada con un filtro de entrada de baja unión a proteínas de 0,22 micras para la perfusión y al finalizar la perfusión se debe lavar la vía con solución inyectable de cloruro de sodio 9 mg/ml (0,9%).
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CYRAMZA 10 MG/ML CONCENTRADO PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 10 mg/mlPrincipio activo: RamucirumabFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 25 mg/mlPrincipio activo: BevacizumabFabricante: Biosimilar Collaborations Ireland LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 25 mg/mlPrincipio activo: BevacizumabFabricante: Mabxience Research S.L.Requiere receta
Médicos online para CYRAMZA 10 MG/ML CONCENTRADO PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CYRAMZA 10 MG/ML CONCENTRADO PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






