CORDIPLAST 5 mg/24 h PARCHES TRANSDERMICOS

Cómo usar CORDIPLAST 5 mg/24 h PARCHES TRANSDERMICOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Cordiplast 5mg/24 h parches transdérmicos
Nitroglicerina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Cordiplast y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cordiplast
- Cómo usar Cordiplast
- Posibles efectos adversos
- Conservación de Cordiplast
- Contenido del envase e información adicional
1. Qué es Cordiplast y para qué se utiliza
Tratamiento preventivo de la angina de pecho, como tratamiento único o en combinación con otros tratamientos antianginosos.
Cordiplast es un parche transdérmico, que contiene nitroglicerina y que sirve para prevenir la angina de pecho, pero no para tratar los ataques agudos.
La nitroglicerina dilata los vasos sanguíneos y mejora el rendimiento del corazón.
Aplicado sobre la piel, Cordiplast libera la nitroglicerina a una velocidad uniforme durante todo el período de aplicación recomendado.
2. Qué necesita saber antes de empezar a usar Cordiplast
No use Cordiplast:
- si es alérgico a la nitroglicerina, a los nitratos o a alguno de los demás componentes de este medicamento, incluidos en la sección 6,
- si padece enfermedades asociadas a un aumento de la presión intracraneal,
- si padece insuficiencia cardiaca debida a obstrucción valvular (estrechamiento de las válvulas del corazón) o a inflamación del pericardio que comprime el corazón,
- si padece cardiomiopatía hipertrófica obstructiva (engrosamiento del corazón que dificulta la circulación),
- en casos de hipotensión grave (presión arterial sistólica inferior a 90 mm Hg),
- en casos de insuficiencia circulatoria aguda asociada con una marcada hipotensión (shock, colapso),
- en casos de hipovolemia grave (disminución del volumen total de sangre),
- en casos de anemia grave,
- en casos de shock cardiogénico (colapso circulatorio de origen cardiaco), a menos que se mantenga una presión final diastólica con las medidas adecuadas,
- en casos de taponamiento cardiaco (compresión aguda del corazón),
- si está tomando medicamentos que contengan sildenafilo, tadalafilo o vardenafilo (medicamento empleado para tratar la impotencia o la hipertensión arterial pulmonar).
- no use Cordiplast simultáneamente con medicamentos que contengan riociguat (medicamento utilizado para el tratamiento de la hipertensión arterial pulmonar y de la hipertensión pulmonar tromboembólica crónica).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Cordiplast.
- si tiene una disminución de la presión de llenado del corazón, p. ej., infarto agudo de miocardio o fallo ventricular izquierdo. Se debe evitar la reducción de la presión sistólica por debajo de 90 mm Hg,
- si padece hipotensión ortostática (bajada de la tensión arterial al levantarse),
- si tiene anemia, consulte con su médico, es posible que Cordiplast no sea un tratamiento adecuado para usted,
- si tiene o ha tenido alguna enfermedad del pulmón o del corazón. Los pacientes con angina de pecho, infarto de miocardio o isquemia cerebral, pueden sufrir alteraciones de las vías respiratorias pequeñas,
- en pacientes con angina de pecho producida por el engrosamiento de su corazón (miocardiopatía hipertrófica), el tratamiento con Cordiplast puede empeorarla,
- existe la posibilidad de un aumento de la frecuencia de angina de pecho durante el intervalo de tiempo sin parche. Puede que su médico considere necesario otro tratamiento antianginoso además de Cordiplast,
- puede darse una pérdida de eficacia por tratamiento continuado con otros medicamentos que contienen nitratos,
- puede producirse un aumento de la metahemoglobina, forma oxidada de la hemoglobina (pigmento de la sangre) que origina metahemoglobinemia.
Cordiplast solo se empleará bajo estricta vigilancia clínica y/o monitorización hemodinámica en pacientes con infarto agudo de miocardio o insuficiencia cardiaca congestiva.
El tratamiento con Cordiplast no se debe interrumpir bruscamente. Su médico le bajará poco a poco la dosis e irá aumentando los intervalos de administración. Si comienza con otro tratamiento para la angina de pecho, durante un periodo de tiempo puede que tenga que utilizar ambos medicamentos.
Cordiplast no contiene aluminio ni otros metales y, por lo tanto, no es necesario retirar el parche antes de una resonancia mágnetica o maniobras para restablecer el ritmo normal del corazón (cardioversión), porque no existe riesgo de quemaduras en la piel por tener el parche adherido.
Niños y adolescentes
La seguridad y eficacia en niños y adolescentes (menores de 18 años) no han sido establecidas.
Uso de Cordiplast con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
El uso de nitratos al mismo tiempo que otros compuestos vasodilatadores, como medicamentos para tratar la hipertensión, inhibidores de la fosfodiesterasa 5, antagonistas del calcio, inhibidores de la ECA, inhibidores de la monoamino-oxidasa, betabloqueantes, diuréticos, algunos antidepresivos y tranquilizantes mayores, pueden bajar excesivamente la tensión arterial.
Los pacientes en tratamiento con Cordiplast nunca (ni aunque se hayan quitado el parche) deben tomar conjuntamente medicamentos inhibidores de la fosfodiesterasa 5 (PDE5) como aquellos que contengan sildenafilo, vardenafilo o tadalafilo (medicamentos usados para tratar la impotencia y la hipertensión arterial pulmonar), ya que podrían producirse complicaciones cardiovasculares que pongan en peligro la vida del paciente. Para mayor información, consulte con su médico o farmacéutico.
Los pacientes que hayan tomado recientemente inhibidores de la fosfodiesterasa (p. ej., sildenafilo, vardenafilo, tadalafilo) no deben ser tratados con soluciones de nitroglicerina en las siguientes 24 horas (48 horas para tadalafilo).
Los medicamentos antiinflamatorios no esteroideos (AINE), excepto el ácido acetilsalicílico (aspirina), pueden reducir la acción de Cordiplast.
La administración con ácido acetilsalicílico (aspirina) puede potenciar la acción de Cordiplast.
El uso al mismo tiempo de Cordiplast con riociguat (medicamento utilizado para el tratamiento de la hipertensión arterial pulmonar y de la hipertensión pulmonar tromboembólica crónica) puede causar hipotensión.
El uso al mismo tiempo de Cordiplast con dihidroergotamina (medicamento utilizado para tratar la migraña), puede aumentar la cantidad de dihidroergotamina en sangre. Esto es importante en pacientes con enfermedad arterial coronaria, ya que la dihidroergotamina contrarresta la acción de Cordiplast, provocando vasoconstricción coronaria.
La administración simultánea de Cordiplast con amifostina (fármaco que se usa para reducir los efectos secundarios no deseados de algunas quimioterapias y radioterapias) y ácido acetilsalicílico puede bajar demasiado la tensión arterial.
La administración simultánea con sapropterina, cofactor de una enzima denominada óxido nítrico sintetasa, puede aumentar el efecto hipotensor de Cordiplast.
Debido al desarrollo de tolerancia a la nitroglicerina, puede disminuir parcialmente el efecto de la nitroglicerina sublingual.
Uso de Cordiplast con los alimentos, bebidas y alcohol
Se debe evitar el uso de alcohol durante el tratamiento con Cordiplast, ya que puede aumentar el efecto hipotensor (disminución de la tensión arterial) y vasodilatador de Cordiplast.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
En caso de embarazo o de sospecha de embarazo, se deberá advertir al médico. Cordiplast debe emplearse con precaución durante el embarazo, especialmente en los 3 primeros meses.
Lactancia
Si se encuentra en periodo de lactancia, deberá advertir al médico, ya que debe decidir si es necesario interrumpir la lactancia o interrumpir el tratamiento, tras considerar el beneficio de la lactancia para el niño y el beneficio del tratamiento para la madre.
Fertilidad
No hay datos disponibles sobre el efecto de la nitroglicerina sobre la fertilidad en humanos.
Conducción y uso de máquinas
Cordiplast puede disminuir la capacidad de reacción o, raramente, puede causar hipotensión ortostática (disminución de la tensión arterial al levantarse) y mareos (como también, excepcionalmente, síncope después de una sobredosis). Los pacientes que sufran alguno de estos efectos deben evitar conducir vehículos o utilizar máquinas.
3. Cómo usar Cordiplast
Siga exactamente las instrucciones de administración de Cordiplast indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
El médico establecerá las dosis adecuadas, tanto al inicio del tratamiento como de mantenimiento, y determinará la frecuencia y duración del mismo. Normalmente, el tratamiento se inicia con un parche al día. En caso necesario, puede aumentarse a 2 parches aplicados simultáneamente, pero solo por recomendación médica. La dosis máxima diaria recomendada es de 20 mg.
El parche deberá aplicarse diariamente sobre la piel por espacio de 12 a 16 horas, asegurando así un periodo libre de nitratos de 8 a 12 horas, respectivamente.
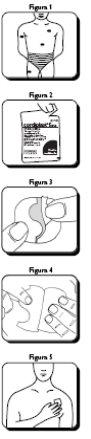
| Para la aplicación de Cordiplast, cualquier zona de la piel que no sea demasiado gruesa y mal irrigada puede ser adecuada, siempre que esté intacta, limpia, relativamente libre de arrugas y sin pelo. Las zonas más recomendables para la aplicación del parche son la parte frontal y lateral del pecho. Sin embargo, también puede aplicarse en el antebrazo, muslo, abdomen y hombro (Figura 1). Para evitar cualquier irritación de la piel, Cordiplast deberá aplicarse en diferentes áreas cada día, procurando no utilizar la misma zona hasta transcurridos, por lo menos, 2-3 días. Cordiplast no deberá aplicarse en la parte distal de las extremidades, en pliegues cutáneos, cicatrices amplias o en áreas quemadas o irritadas. Cordiplast no se adhiere bien a la piel húmeda o sucia, por lo que es importante limpiarla y secarla antes de su aplicación. No utilice productos para el cuidado de la piel antes de la colocación del parche. Cordiplast conserva su función con el baño, la ducha o el ejercicio físico. Cada parche va envasado en una bolsa individual y no debe extraerse de la misma hasta que vaya a utilizarse. La bolsa precintada se rompe fácilmente a través de una hendidura en el borde (Figura 2). Retire el parche de la bolsa y sosténgalo con ambas manos con la lámina protectora hacia arriba. A continuación, baje una mitad del parche, con lo que se abre el corte en forma de S (Figura 3). Seguidamente aplique el parche en el área ya dispuesta y elimine la otra mitad de la capa protectora (Figura 4). Presione a continuación el parche con la mano para asegurar que toda la superficie adhesiva se adhiera firmemente a la piel (Figura 5). Después de la aplicación, lávese bien las manos. |
Los parches de Cordiplast no se deben cortar.
Uso en ancianos: no se requiere ajuste de dosis en pacientes ancianos.
Uso en niños: Cordiplast no está recomendado para uso en niños debido a la ausencia de datos.
Si usa más Cordiplast del que debe
No es probable la aparición de síntomas de sobredosis, debido al tipo de formulación del parche transdérmico de Cordiplast. En caso de que aparecieran, los síntomas pueden ser rápidamente eliminados con la retirada del parche.
Los síntomas son disminución de la presión sanguínea (menor o igual a 90 mm de Hg), palidez, sudoración, pulso débil, aumento de la frecuencia cardiaca (taquicardia), mareo al ponerse de pie, dolor de cabeza (cefalea), colapso, síncope con mareo postural, sensación de debilidad (astenia), náuseas, vómitos y diarrea.
Tras sobredosis accidentales se han descrito casos de metahemoglobinemia (acumulación en la sangre de metahemoglobina, una forma de hemoglobina que no puede transportar oxígeno) que puede provocar cianosis (coloración púrpura de la piel y las mucosas), respiración rápida, ansiedad, pérdida de consciencia e infarto, que puede requerir reanimación inmediata. Aunque con el método de liberación transdérmico es improbable, en dosis altas puede producirse aumento de la presión intracraneal, conduciendo a síntomas cerebrales.
Procedimiento general:
Interrumpir la administración del medicamento, retirando el parche.
Procedimiento general en los acontecimientos de hipotensión relacionada con nitratos.
Se debe colocar al paciente en posición horizontal con las piernas elevadas o, si es necesario, aplicar un vendaje de compresión en las piernas del paciente.
Aporte de oxígeno.
Expansores del volumen plasmático (líquidos intravenosos).
Tratamiento específico para el shock (ingresar al paciente en una unidad de cuidados intensivos).
Procedimientos especiales:
Aumentar la presión sanguínea si esta es muy baja.
Administración adicional de un vasoconstrictor, p. ej. clorhidrato de norepinefrina.
Tratamiento de la metahemoglobinemia:
Reducción del tratamiento de elección con vitamina C, azul de metileno o azul toluidina.
Administración de oxígeno (si es necesario).
Iniciar la respiración artificial.
Hemodiálisis (si es necesario).
El tratamiento de la metahemoglobinemia con azul de metileno está contraindicado en pacientes con una deficiencia de glucosa-6-fosfato o de metahemoglobina-reductasa. Para los casos en que el tratamiento está contraindicado o no es efectivo, se recomienda la realización de una transfusión sanguínea o la transfusión de un concentrado de glóbulos rojos.
Medidas de reanimación:
En caso de paro respiratorio y circulatorio, iniciar las medidas de resucitación inmediatamente.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Cordiplast
No use una dosis doble para compensar las dosis olvidadas. Póngase en contacto con su médico.
Si interrumpe el tratamiento con Cordiplast
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Cordiplast puede producir efectos adversos, aunque no todas las personas los sufran.
Póngase en contacto con su médico inmediatamente si experimenta alguno de los siguientes efectos adversos:
- reacciones alérgicas con hinchazón de la cara, labios, lengua o garganta, dificultades al respirar o tragar, urticaria y picor.
- desmayos, pérdida del conocimiento.
Se detallan a continuación los efectos adversos producidos durante el tratamiento con Cordiplast:
Muy frecuentes (pueden afectar a más de uno de cada 10 pacientes): dolor de cabeza.
Frecuentes (pueden afectar hasta uno de cada 10 pacientes): mareos (incluyendo mareo al ponerse de pie), sensación de mareo, taquicardia, presión arterial baja al ponerse de pie (hipotensión postural), sensación de debilidad (astenia).
Poco frecuentes (pueden afectar hasta uno de cada 100 pacientes): empeoramiento de la angina de pecho (dolor opresivo en la zona del pecho, cuello o brazo), colapso circulatorio (algunas veces acompañado de frecuencia cardiaca lenta y anormal y desmayo), malestar, vómitos, picor, erupciones cutáneas alérgicas, que pueden ser graves, irritación de la piel (picor o quemazón en la zona de aplicación del parche, enrojecimiento, irritación), dermatitis de contacto (que desaparece espontáneamente a las pocas horas de retirar el parche o con el uso de corticoides tópicos).
Muy raros (pueden afectar hasta uno de cada 10.000 pacientes): ardor de estómago.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): palpitaciones, rubor, hipotensión, dermatitis exfoliativa (enfermedad grave con enrojecimiento generalizado de la piel, acompañado de descamación de la piel, y puede incluir picor y caída del cabello), erupción generalizada en la piel, aumento de la frecuencia cardiaca.
Como algunos medicamentos utilizados para tratar enfermedades del corazón, Cordiplast provoca frecuentes dolores de cabeza que dependen de la dosis administrada, debido a la dilatación de los vasos sanguíneos del cerebro. Estos dolores desaparecen después de unos días aunque siga en tratamiento con Cordiplast. Si el dolor de cabeza persiste durante el tratamiento intermitente, se debe tratar con analgésicos suaves. Si, a pesar de ello, los dolores de cabeza persisten, puede que su médico deba bajarle la dosis o retirarle el parche de Cordiplast.
Cualquier ligero enrojecimiento de la piel, normalmente desaparecerá en unas pocas horas tras quitar el parche.
Debe cambiar regularmente el lugar de aplicación para prevenir irritaciones locales.
Puede que su médico le indique otro tratamiento simultáneo para evitar un pequeño aumento de su frecuencia cardiaca.
Se han notificado disminuciones graves de la presión arterial con medicamentos de la misma clase que Cordiplast y pueden incluir náuseas, vómitos, inquietud, palidez y sudoración excesiva.
Durante el tratamiento con estos parches, también puede sufrir un aumento del dolor torácico debido a la falta de oxígeno en el músculo cardiaco y en las zonas alrededor del pecho.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cordiplast
Conservar por debajo de 25ºC.
Conservar en el embalaje original.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deCordiplast5mg/24 h parches transdérmicos
- El principio activo es nitroglicerina. Un parche contiene 18,7 mg de nitroglicerina, en un parche de 9 cm2, que libera 5 mg cada 24 h.
- Los demás componentes son: copolímero de acrilato/acetato de vinilo.
Aspecto del producto y contenido del envase
Envases con 30 parches transdérmicos adhesivos, envasados individualmente en sobres termosellados.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Merus Labs Luxco II S.à.r.l.
208, Val des Bons Malades
L-2121 Luxembourg
Luxemburgo
Responsable de la fabricación
Aesica Pharmaceuticals GmbH
Alfred-Nobel-Str. 10
40789-Monheim (Alemania)
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Norgine de España, S.L.U.
Paseo de la Castellana, 91, 2ª Planta
28046 Madrid
España
Fecha de la última revisión de este prospecto:Mayo 2019
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia7.71 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CORDIPLAST 5 mg/24 h PARCHES TRANSDERMICOSForma farmacéutica: PARCHE TRANSDERMICO, 37,4 mg nitroglicerinaPrincipio activo: Trinitrato de gliceriloFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 31,37 mgPrincipio activo: Trinitrato de gliceriloFabricante: Casen Recordati S.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 47,04 mgPrincipio activo: Trinitrato de gliceriloFabricante: Casen Recordati S.L.Requiere receta
Médicos online para CORDIPLAST 5 mg/24 h PARCHES TRANSDERMICOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CORDIPLAST 5 mg/24 h PARCHES TRANSDERMICOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes