
COMBIPRASAL 0.5 mg/2.5 mg SOLUCION PARA INHALACION POR NEBULIZADOR


Cómo usar COMBIPRASAL 0.5 mg/2.5 mg SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para elusuario
COMBIPRASAL 0.5 mg/2.5 mg
solución para inhalación por nebulizador
Lea todo el prospecto detenidamente antes de empezara usareste medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es COMBIPRASAL y para qué se utiliza
- Qué necesita saber antes de empezar a usar COMBIPRASAL
- Cómo usar COMBIPRASAL
- Posibles efectos adversos
- Conservación de COMBIPRASAL
- Contenido del envase e información adicional
1. Qué es COMBIPRASAL y para qué se utiliza
Este medicamento se presenta en ampollas monodosis que contienen una solución transparente e incolora para inhalación por nebulizador. Combiprasal contiene 0.5 mg de bromuro de ipratropio (como monohidrato) y 2.5 mg de salbutamol (como sulfato).
COMBIPRASAL pertenece a un grupo de medicamentos llamados broncodilatadores que ayudan a abrir los conductos aéreos de los pulmones para que usted pueda respirar más fácilmente.
Está indicado en el tratamiento del broncoespasmo reversible asociado con la enfermedad pulmonar obstructiva crónica (EPOC) en pacientes que requieren más de un único broncodilatador.
2. ANTES DE UTILIZAR COMBIPRASAL
No use COMBIPRASAL
- Si es alérgico (hipersensible) al bromuro de ipratropio, salbutamol sulfato, atropina o a cualquiera de los demás componentes de Combiprasal.
- Si usted padece una enfermedad cardiaca llamada miocardiopatía hipertrófica obstructiva (esto ocurre cuando el corazón no trabaja adecuadamente debido a una inflamación de los músculos cardíacos).
- Si usted tiene un ritmo cardíaco rápido e irregular (lo que se conoce como taquiarritmia).
Advertencias y precauciones
Se ha observado acidosis láctica asociada a altas dosis terapéuticas de salbutamol, principalmente en pacientes tratados con un broncoespasmo agudo (ver las secciones 3 y 4). El aumento en los niveles de lactato puede dar lugar a la falta de respiración e hiperventilación.
Hable inmediatamente con su médico si usted siente que el medicamento no está funcionando como habitualmente y necesita usar el nebulizador más veces de las que su médico le ha recomendado.
Tenga especial cuidado con COMBIPRASAL
Informe a su médico antes de iniciar el tratamiento con este medicamento:
- Si tiene historial de enfermedad cardiaca, ritmo cardíaco irregular o angina de pecho
- Si está embarazada, con intención de estarlo o si está en periodo de lactancia.
- Podrían aparecer reacciones alérgicas inmediatas, tales como urticaria, angioedema, erupción cutánea, tos, pitidos al respirar y dificultad respiratoria (broncoespasmo) e hinchazón de boca y garganta (edema orofaríngeo).
- Cuando se presente dificultad respiratoria aguda que empeora rápidamente, debe consultar inmediatamente a su médico.
- Si padece diabetes.
- Si tiene una glándula tiroide demasiado activa.
- Si tiene un trastorno denominado feocromocitoma, que es un tumor que produce sustancias químicas que pueden causar cansancio, elevada presión sanguínea y ritmo cardíaco más rápido.
- Si padece fibrosis quística, ya que puede ser más propenso a los trastornos de la motilidad gastrointestinal.
- Si usted tiene predisposición a padecer aumento de la presión del ojo (glaucoma de ángulo estrecho). Usted debe evitar que la solución entre en contacto con los ojos. Esto puede provocar dolor o molestia en los ojos, visión borrosa transitoria, halos visuales o imágenes coloreadas, junto con un enrojecimiento de los ojos. En caso de que aparezca cualquiera de estos síntomas, debe interrumpir el tratamiento y consultar inmediatamente a un médico.
- Si padece hiperplasia prostática (aumento del tamaño de la próstata).
- Si tiene un trastorno que dificulte el paso de la orina.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente otros medicamentos, incluso los adquiridos sin receta.
Algunos medicamentos pueden interaccionar con Combiprasal. Es importante que informe a su médico si está tomando alguno de los siguientes medicamentos:
- Medicamentos derivados de la xantina, como la teofilina.
- Medicamentos que contengan beta-bloqueantes tales como el propranolol o el timolol, ya que pueden reducir la eficacia de este medicamento.
- Diuréticos tales como la furosemida o indapamida.
- Digoxina, usada en el tratamiento del fallo cardíaco.
- Otros medicamentos que ayudan a respirar más fácilmente tales como la terbutalina.
- Medicamentos que contengan anticolinérgicos (usados para tratar el asma, síndrome del intestino irritable, enfermedad de Parkinson e incontinencia).
- Ciertos medicamentos para tratar la depresión (conocidos como inhibidores de la monoamino oxidasa y antidepresivos tricíclicos).
- Medicamentos anestésicos, como el halotano.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de tomar cualquier medicamento.
No se recomienda el uso de Combiprasal durante el embarazo o la lactancia. En caso necesario su médico valorará la conveniencia de utilizarlo.
Conducción y uso de máquinas
No se han realizado estudios de los efectos sobre la capacidad para conducir y utilizar máquinas. No obstante, no conduzca o utilice máquinas hasta que sepa cómo tolera el medicamento.
Uso en deportistas
Se informa a los deportistas que este medicamento contiene salbutamol que puede establecer un resultado analítico de control de dopaje como positivo.
3. Cómo usar COMBIPRASAL
Siga exactamente las instrucciones de administración de Combiprasal indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Combiprasal se debe utilizar a demanda y no de forma regular.
Si su asma está activa (por ejemplo, tiene síntomas o crisis frecuentes, como dificultad para respirar que le dificulta hablar, comer o dormir, tos, sibilancias, opresión en el pecho o capacidad física limitada), debe informar inmediatamente a su médico, que le puede empezar a administrar un medicamento o aumentar la dosis de tratamiento, como un corticosteroide inhalado, para controlar su asma.
Informe a su médico lo antes posible si su medicamento parece no estar funcionando tan bien como de costumbre (por ejemplo, si necesita dosis más altas para aliviar sus problemas respiratorios o si su inhalador no le proporciona alivio durante al menos 3 horas), ya que su asma puede estar empeorando y usted puede necesitar un medicamento diferente.
Si utiliza Combiprasal más de dos veces por semana para tratar sus síntomas asmáticos, sin incluir el uso preventivo antes del ejercicio, esto indica un asma mal controlada y puede aumentar el riesgo de ataques de asma graves (empeoramiento del asma) que pueden tener complicaciones serias y pueden poner en riesgo su vida o incluso ser mortales. Se debe poner en contacto con su médico lo antes posible para revisar su tratamiento del asma.
Si utiliza a diario un medicamento contra la inflamación de sus pulmones, p.ej., un “corticosteroide inhalado”, es importante que siga utilizándolo con regularidad, aunque se sienta mejor.
Su médico puede indicarle que use su nebulizador regularmente, a diario o sólo cuando respire con dificultad.
Adultos incluyendo ancianos y niños mayores de 12 años:La dosis recomendada es de 1 envase monodosis, 3 o 4 veces al día. En casos graves, su médico podría incrementar la dosis a 1 envase monodosis, 4 veces al día.
No hay experiencia en la utilización de este medicamento en niños menores de 12 años.
En el caso que no consiga mejoría significativa o que su estado empeore, debe consultar a su médico.
Instrucciones para la correcta administración del preparado:
Su medicación es para inhalación mediante un nebulizador o ventilador, por lo que no debe inyectarse ni tragarse.
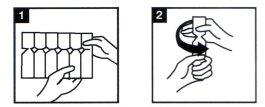
- Prepare su nebulizador siguiendo las instrucciones dadas por el fabricante y su médico. Asegúrese de que el dispositivo nebulizador está limpio.
- Saque de la caja una tira de plástico con ampollas, ábralo y saque una de las ampollas (Fig. 1). Deje el resto de las ampollas en la tira y devuelva ésta a la caja.
- Coja la ampolla y ábrala girando la parte superior (Fig.2).
- A menos que su médico le dé otras indicaciones, añada todo el líquido de la ampolla de plástico en el contenedor de solución del nebulizador.
- Utilice el nebulizador de acuerdo con las instrucciones del médico. Tire la ampolla de plástico vacía.
- Tras usar el nebulizador, límpielo de acuerdo con las instrucciones del fabricante.
Si estima que la acción de Combiprasal es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Si usa más COMBIPRASAL del que debiera:
Si usa más del que debiera, podrían aparecer alteraciones del ritmo cardiaco, palpitaciones, temblor, aumento o descenso de la presión arterial, alteraciones del pulso, angina de pecho, sofocos, sequedad de boca y trastornos de la acomodación visual.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada. Contacte con su médico o Servicio de Urgencias Hospitalarias más cercano. Lleve consigo este prospecto o una ampolla de este medicamento, para que el médico que le trate sepa lo que está tomando.
Si olvidó usar COMBIPRASAL
Si olvidó usar Combiprasal, use la siguiente dosis cuando corresponda o antes en caso de empezar a respirar con dificultad.
No tome una dosis doble para compensar las dosis olvidadas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Combiprasal puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos:
Frecuentes (afectan a 1 de cada 10 personas)
- Nerviosismo
- Dolor de cabeza (cefaleas)
- Tos
- Sequedad de boca
Poco frecuentes (afectan a menos de 1 de cada 100 personas)
- Vértigo
- Temblor
- Disfonía
- Alteraciones del ritmo cardiaco (taquicardia, palpitaciones, arritmia)
- Irritación de garganta
- Náuseas
- Retención urinaria
Raros (afectan a menos de 1 de cada 1000 personas)
- Reacciones alérgicas (hipersensibilidad)
- Disminución de los niveles de potasio en sangre (hipopotasemia)
- Alteraciones psíquicas
- Aumento de la presión intraocular
- Glaucoma de ángulo estrecho
- Dolor ocular
- Dilatación de la pupila (midriasis)
- Obstrucción de las vías respiratorias (broncoespasmo paradójico)
- Vómitos
- Trastornos de la motilidad gastrointestinal
- Reacción cutánea y aumento de la sudoración
- Dolor y debilidad muscular
- Calambres musculares
- Aumento o descenso de la tensión arterial
Los siguientes efectos adversos también pueden ocurrir, pero su frecuencia no es conocida:
- Algunas personas pueden experimentar ocasionalmente dolor en el pecho (debido a problemas de corazón tales como angina de pecho). Avise a su médico si desarrolla estos síntomas mientras está siendo tratado con COMBIPRASAL, pero no deje de tomar este medicamento a menos que así lo indique su médico.
- Una condición conocida como acidosis láctica que puede causar dolor de estómago, hiperventilación, dificultad respiratoria, a pesar de que pueda haber una mejoría en sus sibilancias, pies y manos fríos, latido del corazón irregular o sed.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: http;//www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de COMBIPRASAL
Mantener fuera del alcance y de la vista de los niños.
No conservar a temperatura superior a 25ºC.
Conservar en el embalaje exterior para protegerlo de la luz.
Las ampollas deben abrirse inmediatamente antes de su uso y debe desecharse cualquier resto de solución sobrante.
Caducidad:
No utilice Combiprasal después de la fecha de caducidad que aparece en el envase después de Cad.: La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. INFORMACIÓNADICIONAL
Composición de Combiprasal:
- Los principios activos son bromuro de ipratropio y salbutamol
- Los demás componentes (excipientes) son: cloruro sódico, ácido clorhídrico y agua para inyectables.
Cada ampolla de 2.5 ml de Combiprasal contiene 0.5 mg de bromuro de ipratropio (equivalentes a 0.52 mg de bromuro de ipratropio monohidrato) y 2.5 mg de salbutamol (equivalentes a 3 mg de sulfato de salbutamol).
Aspecto del producto y contenido del envase:
Combiprasal se presenta en tiras de 10 ampollas conteniendo una solución transparente e incolora en el interior de un sobre de aluminio.
Cada caja contiene 20 ampollas.
Titular de la autorización de comercialización:
Laboratorio ALDO-UNIÓN, S.L.
Baronessa de Maldà, 73
08950 Esplugues de Llobregat
Barcelona (España)
Responsable de la fabricación:
Laboratoire Unither
Espace Industriel Nord
151 rue André Durouchez-CS 28028
80084 AMIENS Cedex 2
Francia
Este prospecto ha sido aprobado en Febrero de 2018.
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia13.88 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a COMBIPRASAL 0.5 mg/2.5 mg SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Genetic S.P.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Neutec Inhaler Ireland LimitedRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 0,5 mg/2,5 mgPrincipio activo: salbutamol and ipratropium bromideFabricante: Cipla EuropeRequiere receta
Médicos online para COMBIPRASAL 0.5 mg/2.5 mg SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de COMBIPRASAL 0.5 mg/2.5 mg SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















