
CEVENFACTA 2 MG (90 KIU) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar CEVENFACTA 2 MG (90 KIU) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
CEVENFACTA 1 mg (45 kIU), polvo y disolvente para solución inyectable
CEVENFACTA 2 mg (90 kIU), polvo y disolvente para solución inyectable
CEVENFACTA 5 mg (225 kIU), polvo y disolvente para solución inyectable
eptacog beta (activado)
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es CEVENFACTA y para qué se utiliza
- Qué necesita saber antes de empezar a usar CEVENFACTA
- Cómo usar CEVENFACTA
- Posibles efectos adversos
- Conservación de CEVENFACTA
- Contenido del envase e información adicional
- Instrucciones de uso de CEVENFACTA.
1. Qué es CEVENFACTA y para qué se utiliza
CEVENFACTA contiene el principio activo eptacog beta (activado), un factor VIIa de coagulación recombinante humano (FVIIarh).
CEVENFACTA se usa en adultos y adolescentes (12 años de edad o más) que nacieron con hemofilia A o B y que han desarrollado inhibidores (anticuerpos). Se usa para:
- el tratamiento de episodios de sangrado,
- el manejo de sangrados durante una cirugía.
Cómo actúa CEVENFACTA
Este medicamento actúa formando el coágulo de sangre en el lugar del sangrado, cuando los propios factores de coagulación del cuerpo no funcionan.
2. Qué necesita saber antes de empezar a usar CEVENFACTA
No use CEVENFACTA
- si es alérgico al eptacog beta (activado) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico a los conejos o a las proteínas de conejo.
Advertencias y precauciones
Antes de empezar su tratamiento con CEVENFACTA, informe a su médico:
- Si tiene antecedentes de aterosclerosis (cuando se estrechan las arterias debido a una enfermedad), enfermedad de las arterias coronarias (enfermedad cardiaca debido a un estrechamiento de los vasos sanguíneos que irrigan el corazón), enfermedad cerebrovascular (enfermedad de los vasos sanguíneos que irrigan el cerebro), lesión por aplastamiento, septicemia (infección sanguínea grave) o coágulos de sangre.
- Si tiene una enfermedad cardiaca, una insuficiencia cardiaca o un ritmo cardiaco anormal.
- Si ha tenido alguna vez un coágulo pulmonar (pulmones) o ha sido sometido a una cirugía cardiaca.
- Si padece o ha padecido algún otro problema médico.
Los pacientes con alergia conocida a la caseína pueden tener un mayor riesgo de sufrir reacciones de hipersensibilidad. Si aparecen signos o síntomas de hipersensibilidad, debe interrumpir el tratamiento y acudir inmediatamente al médico. Los síntomas pueden incluir urticaria (hinchazón con prurito bajo la piel), picor, sarpullido, dificultad para respirar, hinchazón alrededor de la boca y la garganta, opresión en el pecho, sibilancias, mareos o desmayos y disminución de la presión arterial.
Aunque no se han observado, en tratamientos con CEVENFACTA se pueden producir las siguientes reacciones:
- Coágulos sanguíneos en las arterias del corazón (que pueden provocar un infarto o una angina de pecho), en el cerebro (que pueden provocar un accidente cerebrovascular) o en los pulmones o las venas profundas. Los síntomas pueden incluir sudoración y dolor en los brazos, las piernas o en abdomen, dolor en el pecho, dificultad para respirar, pérdida de sensibilidad o del movimiento y alteración de la conciencia o del habla.
- Reacciones de hipersensibilidad o anafilácticas. Los síntomas pueden incluir urticaria (hinchazón con prurito bajo la piel), picor, sarpullido, dificultad para respirar, hinchazón alrededor de la boca y la garganta, opresión en el pecho, sibilancias, mareos o desmayos y disminución de la presión arterial.
- Inhibidores (anticuerpos) que pueden provocar problemas de sangrado.
Si presenta alguna de estas reacciones, consulte con su médico antes de seguir utilizando CEVENFACTA.
Es importante registrar el número de lote de su CEVENFACTA. Cada vez que utilice un nuevo envase de CEVENFACTA, anote la fecha y el número de lote (que figura en el envase, después de «Lot») y guarde esa información en un lugar seguro.
Adolescentes
Las advertencias y precauciones indicadas se aplican tanto a los adultos como a los adolescentes (de 12 años de edad o más).
Otros medicamentos y CEVENFACTA
Informe a su médico si ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Debe consultar a su médico antes de usar CEVENFACTA si
- está tomando o ha tomado recientemente otro factor VII activado, concentrados de complejo protrombínico activado o no activado,
- está tomando o ha tomado recientemente factor XIII,
dado que la combinación de estos medicamentos con CEVENFACTA puede aumentar el riesgo de acontecimientos tromboembólicos (formación de coágulos en las venas).
Debe consultar a su médico antes de empezar a usar CEVENFACTA con estos medicamentos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Pueden producirse mareos tras la administración de CEVENFACTA. Debe evitar conducir o usar máquinas mientras experimenta este síntoma.
CEVENFACTA contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por inyección, es decir que esencialmente es «libre de sodio».
3. Cómo usar CEVENFACTA
El uso de este medicamento debe ser iniciado y estar supervisado por un médico con experiencia en el tratamiento de la hemofilia y/o de trastornos hemorrágicos.
CEVENFACTA viene en polvo y se debe preparar (reconstituir) con disolvente e inyectarse en una vena (inyección intravenosa). Consultar las instrucciones de la guía de uso que se encuentra al final de este prospecto (sección 7).
Autoadministración
La inyección de fármacos requiere una formación especial. No intente autoadministrarse a menos que su médico o su centro de tratamiento para la hemofilia le hayan enseñado cómo hacerlo.
Muchas personas con inhibidores aprenden a autoinyectarse ellos mismos o con la ayuda de un familiar. Una vez informado, necesitará un equipo de inyección adicional, aparte de su kit CEVENFACTA, para tratar adecuadamente sus episodios de sangrado en casa. Asegúrese de disponer de todo el equipo de inyección necesario antes de preparar el medicamento para la inyección. Este equipo de inyección adicional le será suministrado por su profesional sanitario (p. ej., su farmacéutico o su centro de tratamiento de la hemofilia).
CEVENFACTA se puede inyectar en un centro de tratamiento de la hemofilia, en la consulta de su médico o en su domicilio. Es importante tratarse a la primera señal de sangrado para poder controlarlo.
Inicie el tratamiento de un sangrado lo antes posible, idealmente en las 2 horas siguientes.
? En caso de sangrado leve o moderado (p. ej., articulación, músculo superficial, tejido blando y membranas mucosas), se debe tratar usted mismo lo antes posible, idealmente en su domicilio.
? En caso de sangrado grave (p. ej., hemorragia de los miembros [brazo o pierna] o con riesgo vital, hemorragia intracraneal [en el cráneo] o gastrointestinal [en el estómago o en el intestino]), se debe poner en contacto con su médico.
Por lo general, los sangrados graves se tratan en el hospital, pero la primera dosis de CEVENFACTA se puede administrar de camino al establecimiento sanitario.
No se trate usted mismo por más de 24 horas sin consultar su médico.
? Cada vez que usa este medicamento, infórme a su profesional sanitario lo antes posible.
? Si el sangrado no se controla en un plazo de 24 horas, póngase en contacto inmediatamente con su profesional sanitario o con el servicio de urgencia. Por lo general, necesitará asistencia hospitalaria.
Para la reconstitución del medicamento antes de la administración, siga la guía de las Instrucciones de usoque se encuentra al final de este prospecto (sección 7).
Inyectar la solución en la vena durante 2 minutos o menos.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Dosis
El profesional sanitario que lo atiende le indicará qué cantidad de CEVENFACTA usar y cuándo administrar el medicamento en función de su peso, su estado y el tipo de sangrado.
Tratamiento de los episodios de sangrado
El tratamiento con este medicamento se debe comenzar tan pronto como se presente el episodio de sangrado.
Sangrados leves y moderados:
El tratamiento a domicilio no debe durar más de 24 horas en caso de episodios de sangrado de leves a moderados. La continuación del tratamiento a domicilio tras 24 horas solo se debe considerar después de una consulta en un centro de tratamiento de la hemofilia.
Sangrados graves:
Busque atención médica inmediata si se presentan signos o síntomas de sangrado grave en casa.
Para evitar cualquier retraso en el tratamiento, la primera dosis se puede administrar de camino al centro para el tratamiento de la hemofilia o a la consulta del médico.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Forma de administración
Para la reconstitución del medicamento antes de la administración y las instrucciones de administración, siga la guía de las Instrucciones de usoque se encuentra al final de este prospecto (sección 7).
Si usa más CEVENFACTA del que debe
Si usa demasiado CEVENFACTA, acuda inmediatamente al médico.
Si olvidó usar CEVENFACTA:
Si olvidó usar CEVENFACTA, consulte a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos frecuentes
(pueden afectar hasta 1 de cada 10 personas)
- Mareos
- Cefalea
- Molestia en el lugar de la inyección
- Cardenales en el lugar de la inyección (hematoma)
- Aumento de la temperatura corporal
- Hematoma postoperatorio
- Reacción relacionada con la inyección
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de CEVENFACTA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 30 ºC.
No congelar.
Conservar el vial en el embalaje exterior para protegerlo de la luz.
Para reconstituir CEVENFACTA, use exclusivamente el material suministrado en el kit.
Tras la reconstitución, el producto se debe conservar en el vial y administrar en un plazo de 4 horas. Toda solución sobrante se debe desechar 4 horas tras la reconstitución.
No utilice este medicamento si observa que el líquido contiene partículas o si es turbio después de mezclar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Conteido del envase e información adicional
Composición de CEVENFACTA
- El principio activo es factor VIIa de coagulación recombinante (eptacog beta (activado))
- Los demás excipientes son:
Polvo: hidrocloruro de arginina, isoleucina, citrato de sodio dihidrato, glicina, hidrocloruro de lisina, polisorbato 80, ácido clorhídrico (para ajuste de pH).
Disolvente: agua para preparaciones inyectables.
Ver sección 2 «CEVENFACTA contiene sodio»
El polvo para solución inyectable contiene: 1 mg/vial (que corresponde a 45 kIU/vial), 2 mg/vial (que corresponde a 90 kIU/vial), 5 mg/vial (que corresponde a 225 kIU/vial).
Tras la reconstitución, la concentración de la solución es de aproximadamente 1 mg/ml (45 kIU/ml) de eptacog beta (activado). 1 kIU es igual a 1 000 UI (Unidades Internacionales).
Aspecto de CEVENFACTA y contenido del envase
El vial de polvo contiene polvo liofilizado de blanco a blancuzco y la jeringa precargada de disolvente contiene una solución transparente e incolora. La solución reconstituida debe ser de transparente a ligeramente opaca.
Cada paquete de CEVENFACTA contiene:
- 1 vial de vidrio con polvo para una solución inyectable,
- 1 adaptador de vial estéril para la reconstitución, equipado con un filtro de 5 µm,
- 1 jeringa precargada con agua para inyecciones,
- 1 vástago con tope trasero.
Presentaciones: 1 mg (45 kIU), 2 mg (90 kIU) y 5 mg (225 kIU).
Titular de la autorización de comercialización
Laboratoire français du Fractionnement et des Biotechnologies
Tour W
102 Terrasse Boieldieu, 19ème Étage
92800 Puteaux
Francia
Fabricante
LFB Biotechnologies
Zone d’activité de Courtab?uf
3 Avenue des Tropiques
91940 Les Ulis
Francia
+33 1 69 82 70 10
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea/Espacio Económico Europeo.
INSTRUCCIONES DE USO
LEA DETENIDAMENTE ESTAS INSTRUCCIONES ANTES DE EMPEZAR A USAR CEVENFACTA
CEVENFACTA viene en forma de polvo. Antes de la inyección, debe prepararse (reconstituirse) con el disolvente suministrado en la jeringa. El disolvente es agua para preparaciones inyectables. El CEVENFACTA reconstituido debe inyectarse en la vena (solo para uso intravenoso).
Este kit proporciona el equipo necesario para reconstituir el medicamento. Se necesitan materiales adicionales para inyectar el medicamento después de la reconstitución. Estos materiales le serán suministrados por su profesional sanitario (p. ej., su farmacéutico o su centro de tratamiento de la hemofilia).
Su médico o su enfermero le mostrarán a usted y/o a su cuidador/a cómo preparar e inyectar CEVENFACTA. No utilice este kit sin una formación adecuada por parte de su profesional sanitario o de su centro de tratamiento de la hemofilia.
Utilice una técnica limpia y libre de gérmenes (aséptica) al preparar e inyectar el medicamento.
Un kit de CEVENFACTA contiene:
- 1 vial de vidrio con polvo para una solución inyectable
- 1 adaptador de vial estéril para la reconstitución, equipado con un filtro de 5 µm
- 1 jeringa precargada con agua para preparaciones inyectables
- 1 vástago con tope trasero

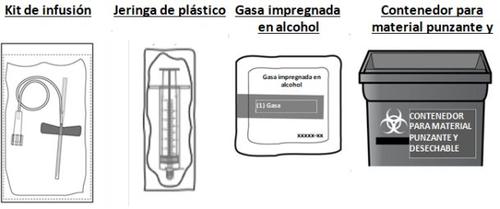
También necesitará un set de inyección estéril (sonda y aguja de mariposa), una jeringa de plástico estéril, gasas esterilizadas impregnadas en alcohol y un contenedor para desechar material punzante que cumpla con las normas y los reglamentos locales aplicables. Estos materiales no están incluidos en el envase de CEVENFACTA.Estos materiales le serán suministrados por su profesional sanitario (p. ej., su farmacéutico o su centro de tratamiento de la hemofilia).
- Reunir el equipo y preparar el vial
- Saque el número de kits de CEVENFACTA que necesite para administrar la dosis prescrita, un set de inyección estéril (no suministrado) y una gasa impregnada en alcohol (no suministrado).
No utilice el kit si el precinto de seguridad se ha roto o si existen señales de que el kit esté contaminado. Utilice uno nuevo en su lugar.
- Compruebe la fecha de caducidad en el lateral del kit (Fig. A).
No lo utilice después de la fecha de caducidad.
- Compruebe el nombre, la concentración y el color de la caja para asegurarse de que contiene el producto correcto (el envase de 1 mg es amarillo, el de 2 mg, verde, y el de 5 mg, morado).
- Trabaje en una superficie limpia y plana antes de iniciar los pasos para la reconstitución de CEVENFACTA.
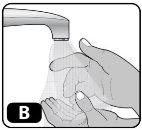
- Lávese las manos con agua y jabón y séquelas con una toalla limpia o al aire (Fig. B).
- Saque el contenido de un kit y una gasa con alcohol. Coloque todo en una superficie limpia (Fig. C).

- Inspeccione todos los elementos contenidos en el kit. Asegúrese de que cada vial tenga una jeringa del mismo color.
No utilice el contenido si se ha caído o está dañado. Utilice un nuevo kit en su lugar.
- Deje que el vial y la jeringa precargada alcancen la temperatura ambiente en caso necesario. Para ello, sosténgalos hasta que los note tan calientes como sus manos.
No utilice el vial y la jeringa precargada calentados de ninguna otra manera.
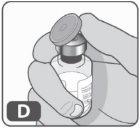
- Retire el tapón de plástico del vial (Fig. D).
Si la tapa de plástico está suelta o el vial viene sin tapa, no lo utilice.
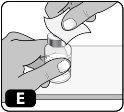
- Limpie el tapón de goma con una gasa impregnada en alcohol (Fig. E)y déjelo secar al aire durante unos segundos para garantizar que está lo más libre de gérmenes posible.
- Tras la limpieza, evite tocar el tapón de goma con los dedos y no deje que entre en contacto con ningún otro objetoantes de colocar el adaptador del vial, ya que esto podría contribuir a la transmisión de gérmenes.
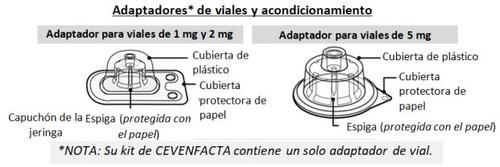
- Colocar el adaptador del vial
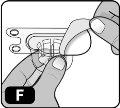
- Despegue la cubierta protectora de papel del envase del adaptador del vial (Fig. F).
Si el papel protector no está completamente sellado o está roto, no utilice el adaptador.
No extraiga el adaptador del vial de su envase protector con los dedos.Si toca con los dedos la espiga del adaptador, puede transmitirle gérmenes.
- Coloque el vial en una superficie limpia y plana y sosténgalo con una mano. Con la otra, coloque la cubierta de plástico (con el adaptador del vial dentro) directamente sobre el vial e inserte la espiga del adaptador en el centro del tapón de goma gris.
- Presione hacia abajo con firmeza para que la espiga del adaptador se inserte en el tapón de goma (es posible que oiga y/o vea cómo encaja) (Fig. G).
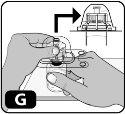
- Retire el envase de plástico del adaptador presionando ligeramente y levantándolo hacia arriba. (Fig. H).
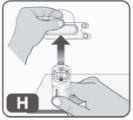
Una vez que ha retirado la cubierta de plástico, no toque la parte superior del adaptador para evitar la transmisión de gérmenes.
NOTA:El adaptador del vial de 5 mg puede no quedar plano en contacto con el vial, pero aun así es perfectamente funcional. Como se ha mencionado anteriormente, su kit CEVENFACTA contiene un solo adaptador de vial (el adecuado para el vial incluido en el kit).
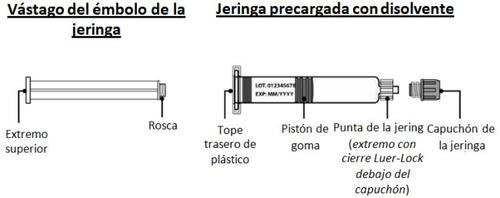
- Colocar la jeringa precargada e introducir el vástago del émbolo
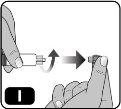
- Retire el capuchón de la jeringa precargada sujetando el cuerpo de la jeringa con una mano y utilizando la otra para desenroscarlo (girando hacia la izquierda) (Fig. I).
Evite tocar la punta de la jeringa para no transmitir los gérmenes de los dedos.
Si el capuchón de la jeringa está suelto o la jeringa venía sin él, no la utilice.
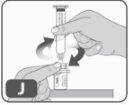
- Sujete los bordes del adaptador del vial y enrosque la jeringa precargada girándola hacia la derecha unas cuantas veces hasta que note resistencia (Fig. J).
Tenga cuidado de no apretar demasiado, ya que luego tendrá que retirar la jeringa.
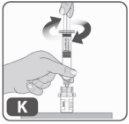
- Para colocar el vástago del émbolo en la jeringa, sujete el extremo superior del vástago del émbolo con una mano y el cuerpo de la jeringa con la otra.
- Introduzca el vástago del émbolo en la jeringa y, a continuación, enrósquelo con unas cuantas vueltas (girando hacia la derecha) para que quede fijado al pistón de goma gris de la jeringa (Fig. K).
- Mezclar el medicamento en el vial
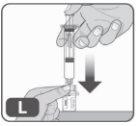
- Empuje muy lentamente el vástago del émbolo hasta el fondo de la jeringa para inyectar todo el líquido de la jeringa en el vial (Fig. L).
No empuje demasiado rápido, ya que podría formarse un exceso de espuma y aire en el vial.
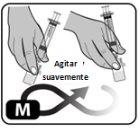
- Gire suavemente el vial o hágalo rodar con cuidado entre las manos para disolver todo el polvo (Fig. M).
No agite el vial, ya que ello puede provocar la formación de espuma y aire.
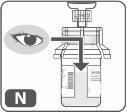
- Revise visualmente la solución final (Fig. N)para asegurarse de que es transparente o ligeramente opaca. Todo el polvo deberá estar disuelto y no deberán quedar partículas flotando en el líquido.
No utilice el producto si la solución contiene cualquier partícula o está turbia después de la mezcla.Repita el proceso con un nuevo kit.
- Retirar la jeringa vacía del adaptador del vial
- Sin volver a introducir ningún medicamento en la jeringa, desenrósquela del adaptador del vial (girando hacia la izquierda) hasta retirarla del todo (Fig. O).
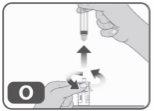
- Deseche la jeringa vacía en un contenedor autorizado para material punzante (Fig. P).
No retire el adaptador del vial.
No toque el extremo superior del adaptador con cierre Luer-Lock. Si lo toca, puede transmitir los gérmenes de los dedos.

- Mezclar el vial (o los viales) extra(s) e inyectar la dosis
- Si su dosis requiere más de un vial, repita los pasos anteriores con kits adicionales hasta obtener la dosis requerida.
- Extraiga el medicamento líquido del vial (o los viales) utilizando una jeringa estéril proporcionada por su farmacia que sea lo suficientemente grande como para contener la dosis prescrita.
- CEVENFACTA debe administrarse dentro de las 4 horas posteriores a la reconstitución (Fig. Q).
No utilizar si han transcurrido más de 4 horas desde la reconstitución.

- CEVENFACTA puede usarse por inyección intravenosa de 2 minutos o menos en la vena, según las indicaciones de su profesional sanitario.
- Desechar los viales vacíos del medicamento
- Tras la reconstitución y la inyección, deseche de forma segura el vial con su adaptador, la jeringa de inyección y cualquier otro material desechable en un contenedor autorizado para material punzante (Fig. R).
No tirar a la basura de residuos domésticos.
No separe el vial del adaptador de vial antes de desecharlos.
No reutilice ninguno de los elementos contenidos en el kit.

Siga los reglamentos y la normativa local para la correcta eliminación del contenedor de material punzante.
Conservación
CEVENFACTA viene en un kit que debe almacenarse por debajo de 30 ºC.
No abra los elementos contenidos en el kit hasta que no esté listo para utilizarlos.
No congele ni conserve en jeringas que contengan la solución de CEVENFACTA reconstituida.
Evite exponer la solución de CEVENFACTA reconstituida a la luz directa.
Información importante
CEVENFACTA solo se inyecta en una vena (administración intravenosa). No lo inyecte por ninguna otra vía como por debajo de la piel (vía subcutánea) o en un músculo (intramuscular). Póngase en contacto con su médico, enfermero o farmacéutico si tiene algún problema.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CEVENFACTA 2 MG (90 KIU) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1 MG (45 kIU)Principio activo: coagulation factor VIIaRequiere recetaForma farmacéutica: INYECTABLE, 5 MG (225 kIU)Principio activo: coagulation factor VIIaRequiere recetaForma farmacéutica: INYECTABLE, 1 mg (50 KUI)Principio activo: coagulation factor VIIaFabricante: Novo Nordisk A/SRequiere receta
Médicos online para CEVENFACTA 2 MG (90 KIU) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CEVENFACTA 2 MG (90 KIU) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















