
CABLIVI 10 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE


Cómo usar CABLIVI 10 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Cablivi 10 mg polvo y disolvente para solución inyectable
caplacizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Cablivi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cablivi
- Cómo usar Cablivi
- Posibles efectos adversos
- Conservación de Cablivi
- Contenido del envase e información adicional
1. Qué es Cablivi y para qué se utiliza
Cablivi contiene el principio activo caplacizumab. Se usa para tratar un episodio de púrpura trombocitopénica trombótica adquiridaen adultos y adolescentes a partir de 12 años que pesan al menos 40 kg. Este es un trastorno raro de la coagulación de la sangre en el que se forman coágulos en los vasos sanguíneos pequeños. Estos coágulos pueden bloquear los vasos sanguíneos y dañar el cerebro, el corazón, los riñones u otros órganos. Cablivi previene la formación de estos coágulos de sangre al impedir que las plaquetas de la sangre se agrupen. De este modo, Cablivi reduce el riesgo de sufrir otro episodio de púrpura trombocitopénica trombótica adquirida (PTTa) poco después del primero.
2. Qué necesita saber antes de empezar a usar Cablivi
No use Cablivi
- si es alérgico a caplacizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Informe a su médico si:
- sangra excesivamente o experimenta síntomas inusuales como dolor de cabeza, dificultad para respirar, cansancio o desmayo que pueden indicar un sangrado interno grave. Su médico puede pedirle que suspenda el tratamiento. El médico le dirá cuándo puede empezar su tratamiento de nuevo.
- está usando medicamentos que previenen o tratan los coágulos sanguíneos como warfarina, heparina, rivaroxabán, apixabán. Su médico decidirá cómo se debe tratar.
- está usando antiplaquetarios como aspirina o heparina de bajo peso molecular (que previenen la formación de coágulos sanguíneos). Su médico decidirá cómo se debe tratar.
- tiene un trastorno hemorrágico, como hemofilia. Su médico decidirá cómo se debe tratar.
- tiene la función del hígado disminuida gravemente. Su médico decidirá cómo se debe tratar.
- va a someterse a una operación o tratamiento dental. Su médico decidirá si se puede posponer o si se debe suspender Cablivi antes de la cirugía o el tratamiento dental.
Niños y adolescentes
No se recomienda Cablivi en niños menores de 12 años y de menos de 40 kg de peso corporal.
Otros medicamentos y Cablivi
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
También informe a su médico si está usando medicamentos anticoagulantes como antagonistas de la vitamina K, rivaroxabán o apixabán, para el tratamiento de los coágulos sanguíneos, o antiplaquetarios como aspirina o heparina de bajo peso molecular, que previenen los coágulos sanguíneos.
Embarazo y lactancia
Informe a su médico si está embarazada o tiene intención de quedarse embarazada. No se recomienda Cablivi durante el embarazo.
Informe a su médico si está en periodo de lactancia. Su médico le aconsejará si es necesario interrumpir la lactancia o no usar Cablivi, teniendo en cuenta el beneficio de la lactancia para el bebé y el beneficio de Cablivi para usted.
Conducción y uso de máquinas
No se espera que Cablivi influya en la capacidad para conducir o utilizar máquinas.
Cablivi contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Cablivi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
El tratamiento con Cablivi lo inicia un médico con experiencia en trastornos sanguíneos.
El tratamientorecomendado es
- primera dosis
- 1 vial inyectado en una vena por un profesional sanitario
- el medicamento se administrará antes de comenzar el intercambio de plasma.
- siguientes dosis
- 1 vial una vez al día como inyección subcutánea (debajo de la piel del abdomen)
- la inyección subcutánea se administrará después de cada intercambio de plasma diario
- después de que termine el intercambio de plasma diario, el tratamiento con Cablivi continuará al menos durante 30 días con una inyección de 1 vial una vez al día
- su médico puede pedirle que continúe el tratamiento diario hasta que se resuelvan los signos subyacentes de su enfermedad.
Su médico puede decidir que usted o su cuidador pongan la inyección de Cablivi. En este caso, su médico o profesional sanitario le entrenará a usted o a su cuidador sobre cómo usar Cablivi.
Instrucciones de uso
La primera inyección de Cablivi en su vena se debe administrar por un profesional sanitario. Las instrucciones para los profesionales sanitarios sobre cómo inyectar Cablivi en la vena se encuentran al final del prospecto.
Para cada inyección, use un envase nuevo para preparar la solución de la inyección. No intente poner la inyección de Cablivi hasta que un profesional sanitario le haya enseñado cómo hacerlo. No use nunca el contenido del envase para otra inyección.
Paso 1- Limpieza
- Lávese bien las manos con agua y jabón.
- Prepare una superficie limpia y plana para colocar el contenido del envase.
- Asegúrese de tener a mano un contenedor para residuos.
Paso 2 -Antes de usar
- Asegúrese de que el envase esté completo.
- Compruebe la fecha de caducidad. No lo use después de la fecha de caducidad.
- No use el envase si los componentes que contiene están dañados de alguna manera.
- Coloque todos los componentes del envase sobre la superficie limpia y plana.
- Si el envase no se conservó a temperatura ambiente, espere a que el vial y la jeringa alcancen la temperatura ambiente (15 °C – 25 °C) dejándolos a temperatura ambiente durante unos minutos. No los caliente de ninguna otra manera.
Paso 3- Desinfección del tapón de goma
- Retire la tapa abatible de plástico del vial. No use el vial si falta la tapa de plástico verde.
- Limpie el tapón de goma expuesto con una de las toallitas con alcohol provistas y deje que se seque durante unos segundos.
- Después de limpiarlo, no toque el tapón de goma ni deje que toque ninguna superficie.
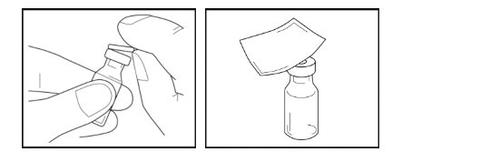
Paso 4- Colocación del adaptador
- Coja el adaptador del vial envasado y retire la cubierta de papel. Deje el adaptador en el envase de plástico abierto. No toque el adaptador.
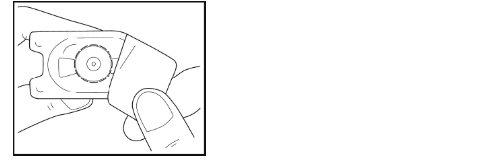
- Coloque el adaptador sobre el vial, mientras mantiene el adaptador en su envase de plástico.
- Presione firmemente hacia abajo hasta que el adaptador encaje en su lugar, con la punta del adaptador atravesando el tapón del vial. Deje el adaptador conectado al vial, todavíaen su envase exterior.
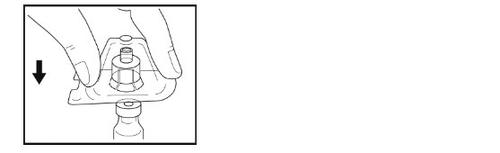
Paso 5- Preparación de la jeringa
- Sosteniendo la jeringa en su mano, rompa la tapa blanca con la otra mano.
- No use la jeringa si la tapa blanca falta, está suelta o está dañada.
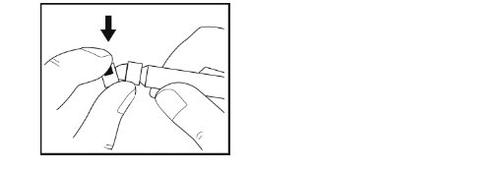
- No toquela punta de la jeringa ni deje que toque ninguna superficie.
- Coloque la jeringa sobre la superficie limpia y plana.
Paso 6– Conexión de la jeringa con el adaptador y el vial
- Tome el vial con el adaptador conectado.
- Retire el envase de plástico del adaptador sujetando el vial con una mano, presionando los lados del envase del adaptador con la otra mano y luego levantando el envase hacia arriba. Tenga cuidado de que el adaptador no se salga del vial.
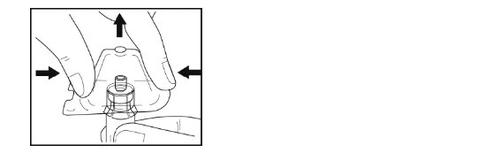
- Sostenga con una mano el adaptador con el vial conectado. Coloque la punta de la jeringa en la parte del conector del adaptador del vial.
- Fije suavemente la jeringa en el vial girándola en el sentido de las agujas del reloj hasta que note resistencia.
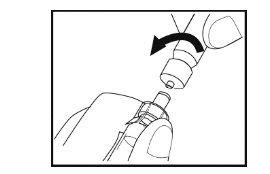
Paso 7- Preparación de la solución
- Mantenga el vial verticalmente en la superficie con la jeringa hacia abajo.
- Empuje lentamente el émbolo de la jeringa hacia abajo hasta que la jeringa esté vacía. No retire la jeringa del vial.
- Con la jeringa todavía conectada al adaptador del vial, gire suavemente el vial con la jeringa conectada hasta que se disuelva el polvo. Evite que se forme espuma. No agiteel vial.
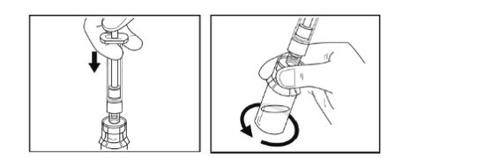
- Deje que el vial con la jeringa conectada permanezca en la superficie durante 5 minutosa temperatura ambiente para permitir que la solución se disuelva por completo. El émbolo puede levantarse por sí solo de nuevo; esto es normal.
- Vaya al paso 8 inmediatamente después de estos 5 minutos.
Paso 8- Extracción de la solución
- Compruebe si la solucióntiene partículas. Se debe haber disuelto todo el polvo y la solución debe ser transparente.
- Presione lentamente el émbolo de la jeringa totalmente hacia abajo.
- Gire por completo hacia abajo el vial, el adaptador y la jeringa.
- Mientras lo mantiene en vertical, tire lentamente del émbolo para poner toda la solución en la jeringa. No lo agite.
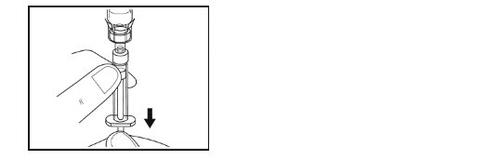
Paso 9 – Preparación de la jeringa para la administración
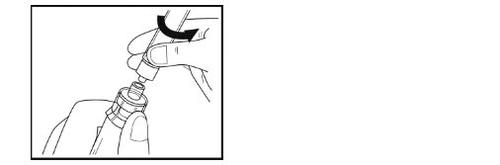
- Gire por completo hacia arriba el vial, el adaptador y la jeringa (con la jeringa en la parte superior). Desconecte la jeringa llena del adaptador sujetando el adaptador con una mano y girando suavemente la jeringa en sentido contrario a las agujas del reloj.
- Coloque el vial y el adaptador conectado en el contenedor para residuos suministrado.
- No toquela punta de la aguja ni permita que toque ninguna superficie. Coloque la jeringa sobre la superficie limpia y plana.
- Vaya al paso 10 para inyectar caplacizumab debajo de la piel del abdomen. Las instrucciones para los profesionales sanitarios sobre cómo inyectar Cablivi en la vena se encuentran al final del prospecto.
Paso 10 -Inserción de la aguja
- Saque la aguja rompiendo la cubierta de papel del envase de la aguja y retirando la aguja con la tapa protectora.
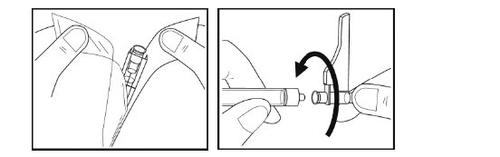
- Sin quitar el capuchón de la aguja, inserte la aguja a la jeringa girando en el sentido de las agujas del reloj hasta que note resistencia.
- Tire hacia atrás el protector de seguridad de la aguja.
- Compruebe el contenido de la jeringa. No utilice el medicamento si observa que está turbio, hay grumos o cualquier otra cosa que no parezca normal. Póngase en contacto con su médico o personal de enfermería si esto sucede.
Paso 11- Preparación del lugar de la inyección para la inyección debajo de la piel
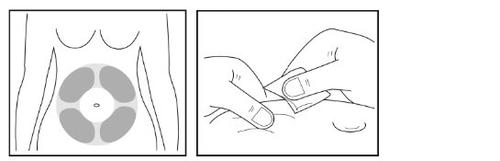
- Seleccione un lugar adecuado (“lugar de la inyección”) en su abdomen para la inyección debajo de la piel.
Evite el área alrededor del ombligo. Seleccione un lugar de inyección diferente del que usó el día anterior para que la piel se pueda recuperar después de la inyección.
- Use la segunda toallita con alcohol para limpiar el lugar de la inyección que haya elegido.
Paso 12- Administración
- Retire con cuidado la tapa de protección de la aguja y tírela. Asegúrese de que la aguja no toque nada antes de la inyección.
- Mantenga la jeringa al nivel de los ojos con la aguja apuntando hacia arriba.
- Elimine las burbujas de aire golpeando el lado de la jeringa con su dedo para que las burbujas suban hacia la punta. Luego, empuje lentamente el émbolo hasta que salga una pequeña cantidad de líquido de la aguja.
- Pellizque suavemente la piel limpia entre los dedos pulgar e índice para hacer un pliegue.
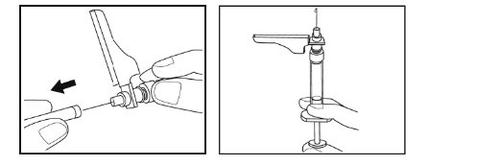
- Mantenga este pliegue de la piel durante toda la inyección.
- Introduzca toda la aguja en el pliegue de la piel con un ángulo como se muestra en la ilustración.
- Presione el émbolo hacia abajo todo lo que pueda.

- Tire de la aguja con el mismo ángulo como la insertó. No frote el lugar de la inyección.
Paso 13- Después de la administración
- Inmediatamente después de la inyección, coloque el protector de seguridad de la aguja sobre la aguja, hasta que encaje en su lugar.
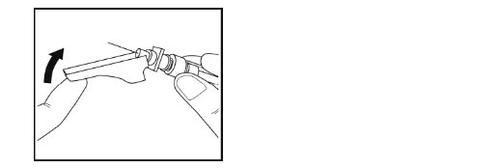
- Ponga la jeringa con la aguja en un contenedor para residuos.
Si usa más Cablivi del que debe
Una sobredosis es poco probable, ya que un vial contiene solo una dosis única. Informe a su médico si cree que ha tenido una sobredosis.
Si olvidó usar Cablivi
Si se olvida de una dosis, debe administrarla si no han pasado más de 12 horas respecto a la hora programada. Si han pasado más de 12 horas desde que se debiera administrar la dosis, no administre la dosis olvidada, pero inyecte la siguiente dosis a la hora habitual.
Si interrumpe el tratamiento con Cablivi
Para obtener el mayor beneficio de su tratamiento, es importante usar Cablivi según se haya recetado y durante el tiempo que su médico le indique que lo use. Informe a su médico antes de suspender el tratamiento, ya que si lo suspende demasiado pronto puede hacer que reaparezca su enfermedad.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Póngase en contacto consu médicoinmediatamentesi se produce alguno de los siguientes efectos adversos graves.
Sangrado prolongado o excesivo.
Su médico puede decidir mantenerle bajo una observación más estrecha o cambiar su tratamiento.
Los efectos adversos en un estudio clínico se notificaron con las siguientes frecuencias:
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- sangrado de las encías
- fiebre
- cansancio
- dolor de cabeza
- sangrados nasales
- sarpullido
Frecuentes:pueden afectar hasta 1 de cada 10 personas
sangrado ocular
- vómitos con sangre
- sangre en las heces
- heces negras y alquitranadas
- sangrado del estómago
- hemorroides sangrantes
- sangrado rectal
- reacciones en el lugar de la inyección: erupción, picazón y sangrado
- sangrado cerebral demostrado por fuertes dolores de cabeza de inicio rápido, vómitos, disminución del nivel de conciencia, fiebre, a veces convulsiones y rigidez de cuello o dolor de cuello
- dolor muscular
- accidente cerebrovascular (ictus)
- sangre en la orina
- sangrado excesivo durante los períodos
- sangrado vaginal
- tos con sangre
- falta de aliento
- moratón
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cablivi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y el envase después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
Conservar en el envase original para protegerlo de la luz.
Cablivi se puede conservar a una temperatura no superior a 25 °C durante un período único de hasta 2 meses, pero no después de la fecha de caducidad. Cablivi no se debe volver a conservar refrigerado después de haberse conservado a temperatura ambiente. No exponer nunca a temperaturas superiores a 30 °C.
No utilice Cablivi si observa partículas o alteración del color antes de la administración.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Contenido de Cablivi
- polvo del vial
- El principio activo es caplacizumab.
Cada vial contiene 10 mg de caplacizumab.
- Los demás componentes son sacarosa, ácido cítrico anhidro, citrato trisódico dihidratado (ver sección 2 “Cablivi contiene sodio”) y polisorbato 80.
- jeringa precargada
La jeringa precargada contiene 1 ml de agua para preparaciones inyectables.
Aspecto de Cablivi y contenido del envase
Cablivi se proporciona como:
- un polvo blanco para solución inyectable en un vial de vidrio, y
- agua para preparaciones inyectables en una jeringa precargada para disolver el polvo.
Después de disolver el polvo en el disolvente, la solución es transparente, incolora o ligeramente amarillenta.
Cablivi está disponible en
- envases individuales que contienen 1 vial con polvo de caplacizumab, 1 jeringa precargada con disolvente, 1 adaptador para el vial, 1 aguja y 2 toallitas con alcohol
- envases múltiples que contienen 7 envases individuales
- envases multidosis que contienen 7 viales con polvo de caplacizumab, 7 jeringas precargadas con disolvente, 7 adaptadores para los viales, 7 agujas y 14 toallitas con alcohol.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y Responsable de la fabricación
Ablynx NV
Technologiepark 21
9052 Zwijnaarde
Bélgica
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Ceská republika Sanofi s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. aus dem Ausland: +49 69 305 70 13 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Ελλ?δα sanofi-aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Österreich sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop IndustrieTél: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536389 | Suomi/Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
La inyección en bolo intravenoso de Cablivi administrada al comienzo del tratamiento se debe administrar por un profesional sanitario. La preparación de una dosis de Cablivi para inyección intravenosa se debe hacer de la misma manera que para una inyección subcutánea (consulte las Instrucciones de uso, paso 1 a 9, en la sección 3).
Cablivi se puede administrar por vía intravenosa conectando la jeringa preparada a conexiones Luer lock estándar de líneas intravenosas o usando una aguja adecuada. La línea se puede lavar con solución inyectable de cloruro de sodio 9 mg/ml (0,9 %).
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CABLIVI 10 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1,5 mgPrincipio activo: fondaparinuxFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: INYECTABLE, 2,5 mgPrincipio activo: fondaparinuxFabricante: Viatris Healthcare LimitedRequiere recetaForma farmacéutica: INYECTABLE, 5 mgPrincipio activo: fondaparinuxFabricante: Viatris Healthcare LimitedRequiere receta
Médicos online para CABLIVI 10 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CABLIVI 10 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















