BUPRENORFINA TEVA 70 MICROGRAMOS/HORA PARCHE TRANSDERMICO EFG

Cómo usar BUPRENORFINA TEVA 70 MICROGRAMOS/HORA PARCHE TRANSDERMICO EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Buprenorfina Teva 70 microgramos/hora parche transdérmico EFG
Buprenorfina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Buprenorfina Teva y para qué se utiliza
- Qué necesita saber antes de empezar a usar Buprenorfina Teva
- Cómo usar Buprenorfina Teva
- Posibles efectos adversos
- Conservación de Buprenorfina Teva
- Contenido del envase e información adicional
1. Qué es Buprenorfina Teva y para qué se utiliza
La sustancia activa de Buprenorfina Teva es buprenorfina.
Buprenorfina Teva es un analgésico (un medicamento para el alivio del dolor) indicado para el alivio del dolor moderado a severo oncológico y del dolor severo que no responda a otros tipos de analgésicos. Buprenorfina Teva actúa a través de la piel. Cuando se aplica el parche transdérmico sobre la piel, el principio activo buprenorfina pasa a través de la piel hasta la sangre. La buprenorfina es un opioide (medicamento para el alivio del dolor intenso) que reduce el dolor actuando sobre el sistema nervioso central (en células nerviosas específicas en la médula espinal y en el cerebro). El efecto del parche transdérmico dura hasta un máximo de cuatro días. Buprenorfina Teva no es idóneo para el tratamiento del dolor agudo (de corta duración).
2. Qué necesita saber antes de empezar a usar Buprenorfina Teva
No use Buprenorfina Teva
- si es alérgico a la buprenorfina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6),
- si es adicto a analgésicos potentes (opioides),
- si padece una enfermedad en la que tiene gran dificultad para respirar o en la que esto puede ocurrirle,
- si está tomando inhibidores de la MAO (ciertos medicamentos para el tratamiento de la depresión) o los ha tomado en las dos últimas semanas antes del tratamiento con Buprenorfina Teva (ver “Otros medicamentos y Buprenorfina Teva”),
- en caso de miastenia gravis (un tipo de debilidad muscular severa),
- en caso de delirium tremens (confusión y temblor causados por la abstinencia de alcohol tras una ingesta excesiva habitual del mismo o durante un episodio de consumo elevado de alcohol),
- en caso de embarazo.
Buprenorfina Teva no se debe utilizar para tratar el síndrome de abstinencia en drogodependientes.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Buprenorfina Teva
- si ha bebido mucho alcohol recientemente,
- si tiene crisis epilépticas o convulsiones (ataques),
- si tiene la consciencia alterada (sensación de mareo o desmayo), por causa desconocida,
- si está en estado de shock (un signo podría ser el sudor frío),
- si tiene la presión craneal elevada (por ejemplo después de traumatismo craneoencefálico o en enfermedad cerebral), sin la posibilidad de respiración artificial,
- si tiene dificultad para respirar o está tomando otra medicación que puede hacerle respirar más lenta o débilmente (ver “Otros medicamentos y Buprenorfina Teva”),
- si tiene depresión u otras enfermedades que se tratan con antidepresivos.
El uso de estos medicamentos junto con Buprenorfina Teva puede provocar sindrome serotoninérgico, una enfermedad potencialmente mortal (ver «Otros medicamentos y Buprenorfina Teva»),
- si tiene problemas hepáticos,
- si tiene tendencia al abuso de medicamentos o drogas.
Tenga en cuenta también las siguientes precauciones:
- Algunas personas pueden llegar a depender de analgésicos potentes tales como Buprenorfina Teva cuando los utilizan durante mucho tiempo. Estos pacientes pueden tener efectos después de que dejen de utilizarlos (ver “Si interrumpe el tratamiento con Buprenorfina Teva”).
- La fiebre y el calor ambiental pueden dar lugar a cantidades mayores que las normales de buprenorfina en sangre. También, el calor ambiental puede impedir que el parche transdérmico se pegue adecuadamente. Por lo tanto, consulte a su médico si tiene fiebre y no se exponga a fuentes de calor (ej.: sauna, lámparas infrarrojas, mantas eléctricas o bolsas de agua caliente).
- Trastornos respiratorios relacionados con el sueño: Buprenorfina Teva puede causar trastornos respiratorios relacionados con el sueño tales como apnea de sueño (pausas respiratorias durante el sueño) e hipoxemia relacionada con el sueño (niveles bajos de oxígeno en sangre). Los síntomas pueden incluir pausas respiratorias durante el sueño, despertares nocturnos debidos a la dificultad para respirar, dificultad para mantener el sueño o somnolencia excesiva durante el día. Contacte con su médico si usted u otra persona observa estos síntomas. Su médico podría considerar una reducción de la dosis.
- Se debe advertir a los deportistas que este medicamento puede dar un resultado positivo en las pruebas de control del dopaje.
Tolerancia, dependencia y adicción
Este medicamento contiene buprenorfina, una sustancia opioide. El uso repetido de opioides puede disminuir la eficacia del medicamento (su organismo se acostumbra al medicamento, esto es lo que se conoce como tolerancia). El uso repetido de Buprenorfina Teva también puede causar dependencia, abuso y adicción, lo que puede dar lugar a una sobredosis potencialmente mortal. El riesgo de efectos adversos puede aumentar con una dosis más alta y una duración de uso más prolongada. La dependencia o la adicción pueden hacerle sentir que ya no tiene el control de la cantidad de medicamento que necesita tomar o con qué frecuencia debe tomarlo.
El riesgo de volverse dependiente o adicto varía según la persona. Puede presentar un mayor riesgo de volverse dependiente o adicto a Buprenorfina Teva si:
- Usted o algún miembro de su familia tiene antecedentes de abuso o dependencia del alcohol, medicamentos de venta con receta o sustancias ilícitas (“adicción”).
- Es fumador.
- Alguna vez ha tenido problemas con su estado de ánimo (depresión, ansiedad o un trastorno de la personalidad) o ha recibido tratamiento de un psiquiatra para otras enfermedades mentales.
Si nota alguno de los siguientes signos mientras toma Buprenorfina Teva podría ser una señal de que se ha vuelto dependiente o adicto:
- Necesita tomar el medicamento durante más tiempo del recomendado por su médico.
- Necesita tomar más dosis de la recomendada.
- Es posible que sienta que necesita seguir usando su medicamento, incluso cuando no le ayude a aliviar el dolor.
- Está usando el medicamento por razones distintas a las prescritas, por ejemplo, “para calmarse” o “para ayudarle a dormir”.
- Ha hecho intentos repetidos y sin éxito de dejar o controlar el uso del medicamento.
- No se encuentra bien cuando deja de tomar el medicamento y se siente mejor cuando vuelve a tomarlo (“síntomas de abstinencia”).
Si nota alguno de estos signos, hable con su médico para abordar la estrategia terapéutica más adecuada en su caso, incluido cuándo es apropiado dejar de tomarlo y cómo hacerlo de forma segura (ver sección 3 “Si interrumpe el tratamiento con Buprenorfina Teva”).
Niños y adolescentes
Buprenorfina Teva no se debe utilizar en personas menores de 18 años, porque no se tiene experiencia hasta el momento en este grupo de edad.
Otros medicamentos y Buprenorfina Teva
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
- Buprenorfina Teva no se debe utilizar junto con inhibidores de la MAO (ciertos medicamentos para el tratamiento de la depresión), o si los ha tomado en las dos últimas semanas.
- Buprenorfina Teva puede producir en algunos pacientes somnolencia, vómitos, mareos o hacerles respirar más lenta o débilmente. Estos efectos adversos pueden intensificarse si se toman al mismo tiempo otros medicamentos que pueden producir los mismos efectos. Estos otros medicamentos incluyen otros analgésicos potentes (opioides), ciertos medicamentos para dormir, anestésicos y medicamentos para el tratamiento de ciertas enfermedades psicológicas como tranquilizantes, antidepresivos y neurolépticos.
El uso concomitante de Buprenorfina Teva y medicamentos sedantes como benzodiazepinas o medicamentos relacionados aumenta el riesgo de somnolencia, dificultad para respirar (depresión respiratoria), coma y puede ser potencialmente mortal. Debido a esto, el uso concomitante solo se debe considerar cuando otras opciones de tratamiento no son posibles.
Sin embargo, si su médico le prescribe Bupernorfina Teva junto con medicamentos sedantes, su médico debe limitar la dosis y la duración del tratamiento concomitante.
Informe a su médico sobre todos los medicamentos sedantes que esté tomando y siga de cerca la recomendación de la dosis. Podría ser útil informar a amigos o familiares para que estén al tanto de los signos y síntomas indicados anteriormente. Contacte con su médico cuando experimente tales síntomas.
El uso concomitante de Buprenorfina Teva y gabapentinoides como gabapentina o pregabalina utilizados para tratar la epilepsia o el dolor debido a problemas nerviosos (dolor neuropático) puede provocar dificultades para respirar (depresión respiratoria), presión arterial baja, somnolencia profunda, coma y puede ser potencialmente mortal.
- Si Buprenorfina Teva se utiliza junto con anticolinérgicos o medicamentos con actividad anticolinérgica, como medicamentos para tratar la depresión, medicamentos utilizados para tratar alergias, mareos o náuseas (antihistamínicos o antieméticos), medicamentos para tratar trastornos psiquiátricos (antipsicóticos o neurolépticos), relajantes musculares o medicamentos para tratar la enfermedad de Parkinson, los efectos secundarios anticolinérgicos pueden aumentar.
- Si Buprenorfina Teva se utiliza junto con algunos medicamentos, la acción del parche transdérmico se puede intensificar. Estos medicamentos incluyen por ejemplo ciertos antiinfecciosos y antifúngicos (ej.: aquellos que contienen eritromicina o ketoconazol) o medicamentos para el VIH (ej.: aquellos que contienen ritonavir).
- Si Buprenorfina Teva se utiliza junto con otros medicamentos, la acción del parche transdérmico puede reducirse. Estos medicamentos incluyen por ejemplo dexametasona, ciertos productos para el tratamiento de la epilepsia (ej.: aquellos que contienen carbamazepina o fenitoína) o medicamentos que se utilizan para el tratamiento de la tuberculosis (ej.: rifampicina).
- Algunos medicamentos pueden aumentar los efectos secundarios de Buprenorfina Teva y en ocasiones pueden provocar reacciones muy graves. No tome ningún otro medicamento mientras esté tomando Buprenorfina Teva sin consultar primero a su médico, especialmente antidepresivos como moclobemida, tranilcipromina, citalopram, escitalopram, fluoxetina, fluvoxamina, paroxetina, sertralina, duloxetina, venlafaxina, amitriptilina, doxepina o trimipramina. Estos medicamentos pueden interactuar con Buprenorfina Teva y puede experimentar síntomas como contracciones musculares rítmicas involuntarias, incluidos los músculos que controlan el movimiento de los ojos, agitación, alucinaciones, coma, sudoración excesiva, temblores, exageración de los reflejos, aumento de la tensión muscular, temperatura corporal superior a 38 °C. Póngase en contacto con su médico si sufre estos síntomas.
Uso de Buprenorfina Teva con alimentos, bebidas y alcohol
No debe beber alcohol mientras utilice Buprenorfina Teva. El alcohol puede intensificar ciertos efectos adversos del parche transdérmico y puede que no se encuentre bien. Si usted bebe zumo de pomelo puede intensificar los efectos de Buprenorfina Teva.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No existe experiencia suficiente del uso de Buprenorfina Teva hasta el momento en mujeres embarazadas. Por lo tanto, no utilice Buprenorfina Teva durante el embarazo.
Buprenorfina, el principio activo contenido en el parche transdérmico, inhibe la producción de leche y pasa a la leche materna. Por lo tanto, no utilice Buprenorfina Teva durante la lactancia.
Conducción y uso de máquinas
Buprenorfina Teva puede hacer que se sienta mareado, somnoliento o tener visión doble o borrosa y puede alterar sus reflejos de forma que no reaccione adecuadamente o lo suficientemente rápido en el caso de situaciones súbitas o inesperadas. Esto se aplica especialmente:
- al principio del tratamiento
- cuando cambie la dosis
- cuando cambie de otro medicamento a Buprenorfina Teva
- si usted toma también otros medicamentos que actúan en el cerebro
- si usted bebe alcohol
Si está afectado, no debería conducir o manejar máquinas mientras utiliza Buprenorfina Teva. Esto también se aplica al final del tratamiento con Buprenorfina Teva. No conduzca o maneje máquinas por lo menos durante las 24 horas posteriores a la retirada del parche.
En caso de duda consulte con su médico o farmacéutico.
3. Cómo usar Buprenorfina Teva
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Antes de iniciar el tratamiento y de forma periódica durante el mismo, su médico hablará con usted sobre lo que puede esperar del uso de este medicamento, cuándo y durante cuánto tiempo debe tomarlo, cuándo ponerse en contacto con su médico y cuándo debe dejar de tomarlo (ver también “Si interrumpe el tratamiento con Buprenorfina Teva”).
Este medicamento se encuentra disponible en tres dosis: Buprenorfina Teva 35 microgramos/hora parche transdérmico EFG, Buprenorfina Teva 52,5 microgramos/hora parche transdérmico EFG y Buprenorfina Teva 70 microgramos/hora parche transdérmico EFG.
Su médico ha elegido este parche de buprenorfina, como el más adecuado para usted. Durante el tratamiento, su médico puede cambiar el parche transdérmico que utiliza por otro de dosis menor o mayor si es necesario.
La dosis recomendada es:
Adultos
Siga estas instrucciones a menos que su médico le haya dado otras indicaciones distintas. Aplíquese el parche de buprenorfina (como se detalla abajo) y cámbieselo después de cuatro días, como máximo. Para facilitar su uso, puede cambiar el parche 2 veces a la semana en días fijos, por ej.: “siempre los lunes por la mañana y los jueves por la tarde”. Para ayudarle a recordar cuándo debe cambiar el parche transdérmico, anótelo en el calendario del cartonaje. Si su médico le ha indicado que tome otros analgésicos además del parche transdérmico, siga estrictamente las instrucciones de su médico, si no usted no se beneficiará completamente del tratamiento con buprenorfina.
Niños y adolescentes
Este medicamento no se debe utilizar en personas menores de 18 años porque hasta el momento no se tiene experiencia en ese grupo de edad.
Pacientes de edad avanzada
No se requiere ajuste de la dosis en pacientes de edad avanzada.
Pacientes con alteraciones renales / pacientes sometidos a diálisis
En pacientes con alteración renal y pacientes sometidos a diálisis, no se necesita ajuste de la dosis.
Pacientes con alteración hepática
En pacientes con alteración hepática, la intensidad y duración de la acción de buprenorfina puede verse afectada. Si usted pertenece a este grupo de pacientes, su médico se lo controlará con un mayor cuidado.
Método de administración
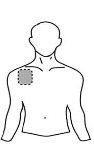
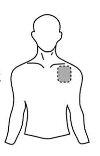
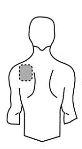
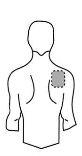
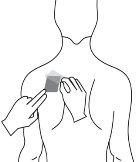
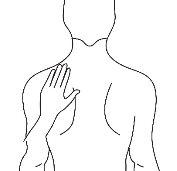
Antes de aplicar un parche transdérmico
| Pecho |
|
|
Espalda |
|
|
- Si la zona elegida tiene vello, córtelo con unas tijeras. ¡No lo afeite!
- Evite áreas de la piel enrojecidas, irritadas o que tengan cualquier otro tipo de manchas, por ejemplo grandes cicatrices.
- La zona de la piel que elija debe estar seca y limpia. Si fuera necesario lávela con agua fría o tibia. No utilice jabón u otros detergentes. Después de un baño caliente o ducha espere hasta que su piel esté completamente seca y fría. No aplique lociones, cremas o pomadas en el área elegida. Esto podría impedir que el parche transdérmico se pegue adecuadamente.
Aplicación del parche transdérmico:
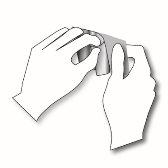
Paso 1: Cada parche transdérmico está precintado en un sobre. Justo antes de su uso, corte el sobre a lo largo del borde sellado con unas tijeras. Tenga cuidado en no dañar los parches transdérmicos. Coja el parche transdérmico. |
|
Paso 2: El lado adhesivo del parche transdérmico está cubierto por una lámina protectora transparente. Despegue cuidadosamente la mitadde la lámina. Intente no tocar la parte adhesiva del parche transdérmico. |
|
Paso 3: Pegue el parche transdérmico en el área de la piel que haya elegido y retire el resto de la lámina. |
|
Paso 4: Presione el parche transdérmico contra su piel con la palma de su mano y cuente lentamente hasta 30. Asegúrese que todo el parche transdérmico está en contacto con su piel, especialmente los bordes. |
|
Paso 5: Lave sus manos después de usar el parche transdérmico. No use ningún producto de limpieza. | |
Mientras lleve el parche transdérmico
Puede llevar el parche transdérmico como máximo 4 días. Si se ha aplicado el parche transdérmico correctamente, el riesgo de que se despegue es bajo. Usted puede ducharse, bañarse o nadar mientras lo lleva. Sin embargo, no exponga el parche transdérmico a calor extremo (ej.: sauna, lámparas infrarrojas, mantas eléctricas o bolsas de agua caliente).
En el caso improbable de que su parche transdérmico se cayera antes de que necesitara cambiarse, no utilice el mismo parche transdérmico de nuevo. Pegue uno nuevo inmediatamente (ver “Cambio del parche transdérmico” debajo).
Cambio del parche transdérmico
- Retire con cuidado el parche viejo.
- Dóblelo por la mitad con el lado adhesivo hacia dentro.
- Deséchelo con precaución, fuera de la vista y del alcance de los niños.
- Pegue un nuevo parche transdérmico sobre una zona distinta de la piel (como se ha descrito antes). Debe transcurrir al menos 1 semana antes de poder aplicar un parche nuevo en la misma área de la piel.
Duración del tratamiento
Su médico le indicará la duración de su tratamiento con este medicamento. No suspenda el tratamiento con buprenorfina por su cuenta, ya que el dolor puede volver a aparecer y puede sentirse mal (ver también “Si interrumpe el tratamiento con Buprenorfina Teva”).
Si estima que el efecto de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Si usa más Buprenorfina Teva del que debe
Si esto ocurre pueden existir signos de una sobredosificación por buprenorfina. Una sobredosificación puede intensificar los efectos adversos de buprenorfina tales como somnolencia, náuseas y vómitos. Puede tener las pupilas puntiformes y la respiración puede llegar a ser lenta y débil. Usted también podría sufrir un colapso cardiovascular.
Tan pronto como se dé cuenta de que ha utilizado más parches transdérmicos de los que debe, quítese los parches transdérmicos en exceso y consulte inmediatamente a su médico o a su farmacéutico.
En caso de sobredosis o ingestión accidental, consulte al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad utilizada
Si olvidó usar Buprenorfina Teva
Si usted olvidó una aplicación, pegue un parche transdérmico nuevo tan pronto como se dé cuenta. Esto hará que usted cambie su rutina, por ej.: si normalmente se aplicaba su parche transdérmico los lunes y los jueves, pero lo olvidó y no se puso el nuevo parche transdérmico hasta el miércoles, a partir de ahora necesitará cambiar sus parches transdérmicos los miércoles y los sábados. Anote el nuevo par de días en el calendario del cartonaje. Si cambia el parche transdérmico demasiado tarde, el dolor puede aparecerle de nuevo. En este caso consulte con su médico.
¡Nunca se aplique más de un parche transdérmico para compensar el que se olvidó!
Si interrumpe el tratamiento con Buprenorfina Teva
Si interrumpe o finaliza el tratamiento con este medicamento demasiado pronto, el dolor le reaparecerá. Si desea suspender el tratamiento debido a los efectos adversos desagradables, consulte a su médico. Su médico le dirá lo que puede hacer y si puede tratrarse con otros medicamentos.
Algunas personas pueden experimentar efectos de abstinencia después de haber utilizado analgésicos potentes durante mucho tiempo, al dejar de utilizarlos. El riesgo de tener estos efectos después de suspender la aplicación de los parches de buprenorfina es muy bajo. Sin embargo, si se siente agitado, ansioso, nervioso, o tembloroso, si está hiperactivo, tiene dificultad para dormir o problemas de digestión, consulte con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta inflamación de las manos, pies, rodillas, cara, labios, boca o garganta, la cual puede causar dificultad al tragar o respirar, habones urticariales, desvanecimiento, color amarillento de la piel y ojos (también llamada ictericia), quítese el parche transdérmico y consulte con su médico o acuda al hospital más próximo inmediatamente. Éstos pueden ser síntomas de una reacción alérgica grave muy rara.
Se ha informado de los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- náuseas (sentirse enfermo)
- eritema, prurito
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- mareo, dolor de cabeza
- falta de aliento
- vómitos, estreñimiento
- cambios en la piel (exantema, generalmente por uso repetido), aumento de la sudoración
- edema (tumefacción de las piernas), cansancio
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- confusión, trastornos del sueño, inquietud
- diferentes grados de sedación (serenidad), que van desde cansancio a confusión
- trastornos circulatorios (tales como hipotensión o raramente, pérdida de conocimiento debido a la caída de la tensión arterial)
- sequedad de boca
- erupciones
- retención urinaria (menos orina de lo normal), alteraciones de la micción
- debilidad (abatimiento)
Raros(pueden afectar hasta 1 de cada 1.000 personas)
- Pérdida de apetito
- ilusiones como alucinaciones, ansiedad, pesadillas, disminución del deseo sexual
- dificultad en la concentración, trastornos del habla, confusión, alteraciones del equilibrio, sensaciones anormales en la piel (sensación de calor, hormigueo o entumecimiento)
- alteraciones de la visión, visión borrosa, hinchazón de los párpados
- sofocos
- dificultad para respirar (depresión respiratoria)
- acidez de estómago
- habón urticarial
- dificultades en la erección
- síntomas de abstinencia, reacciones en el lugar de la administración
Muy raros(pueden afectar hasta 1 de cada 10.000 personas)
- reacciones alérgicas graves
- dependencia, cambios de humor
- contracción muscular, alteraciones del gusto
- pupilas pequeñas
- dolor de oído
- hiperventilación, hipo
- arcadas
- pústulas, vesículas
- dolor en el pecho
Frecuencia no conocida(la frecuencia no puede estimarse a partir de los datos disponibles)
- dermatitis de contacto (erupción cutánea con inflamación que puede incluir sensación de quemazón), decoloración de la piel
Si nota alguno de los efectos adversos anteriormente mencionados, consulte con su médico lo antes posible.
En algunos casos tienen lugar reacciones alérgicas locales tardías con marcados signos de inflamación. En estos casos se debe interrumpir el tratamiento con Buprenorfina Teva, después de haberlo consultado con su médico.
Algunas personas pueden tener síntomas de retirada del tratamiento tras haber usado analgésicos potentes por un período de tiempo prolongado y dejar de utilizarlos. Después del tratamiento con Buprenorfina Teva, el riesgo de padecer síntomas de abstinencia es bajo. Sin embargo, si siente agitación, ansiedad, nerviosismo, hiperactividad, trastornos en el sueño, o problemas digestivos, consulte a su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Buprenorfina Teva
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el sobre después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Conserve este medicamento en un lugar seguro y protegido, donde otras personas no puedan acceder a él. Puede causar daños graves y ser mortal para las personas que pudieran usarlo de manera accidental o intencionada cuando no se les ha recetado.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Buprenorfina Teva
El principio activo es buprenorfina.
Cada parche transdérmico de 50 cm2 contiene 40 mg de buprenorfina y libera 70 microgramos de buprenorfina por hora.
Los demás componentes son:
- Matriz adhesiva (con buprenorfina) povidona K90, ácido levulínico, oleil oleato, poli[ácido acrílico-co-butilacrilato-co-(2-etilhexil)acrilato-co-vinilacetato] (5:15:75:5)
- Matriz adhesiva (sin buprenorfina): poli[(2-etilhexil)acrilato-co-glicidilmetacrilato-co-(2-hidroxietil)acrilato-co-vinilacetato] (68:0,15:5:27)
- Lámina separadora entre las matrices adhesivas con y sin buprenorfina: lámina poli(etilentereftalato).
- Capa de recubrimiento: poliéster.
- Lámina protectora de liberación (en la parte anterior cubriendo la matriz adhesiva que contiene buprenorfina): lámina de poli(etilentereftalato) siliconada.
- Tinta de impresión azul
Aspecto de Buprenorfina Tevay contenido del envase
Parche rectangular con los bordes redondeados de color beige e impreso “Buprenorfina” y “70 μg / h”
Cada parche transdérmico está sellado en un sobre a prueba de niños. Los parches están disponibles conteniendo 3, 4, 5, 6, 8, 10, 12, 16, 18 o 20 parches transdérmicos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Teva Pharma S.L.U.
C/ Anabel Segura, 11 Edificio Albatros B, 1ª planta
28108 Alcobendas (Madrid)
España
Responsable de la fabricación
Labtec GmbH
Heykenaukamp 10, Hamburgo
21147 Alemania
o
Merckle GmbH
Ludwig-Merckle-Straße 3, Blaubeuren
89143 Alemania
o
Teva Operations Poland Sp.z.o.o
ul. Mogilska 80, Krakow
31-546 Polonia
Este medicamento está autorizado en los Estados Miembros con los siguientes nombres:
Alemania: | Buprenoratiopharm 70 Mikrogramm/Stunde Transdermales Pflaster |
Austria: | Buprenorphin ratiopharm 70 Mikrogramm/h transdermales Pflaster |
Bélgica: | Buprenorphine Teva 70 microgram/u pleister voor transdermaal gebruik Buprenorphine Teva 70 microgrammes/h dispositif transdermique Buprenorphine Teva 70 Mikrogramm/S transdermales Pflaster |
España: | Buprenorfina Teva 70 microgramos/hora parche transdérmico EFG |
Finlandia: | Buprenorphine ratiopharm 70 mikrog/tunti depotlaas-tari |
Croacia: | Laribon 70 mikrograma/h transdermalni flaster |
Paises Bajos: | Buprenorfine Teva 70 microgram/uur pleister voor transdermaal gebruik |
Portugal: | Buprenorfina ratiopharm |
Reino Unido: | Timpron 70 micrograms/h Transdermal patch |
Fecha de la última revisión de este prospecto:octubre 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/80611/P_80611.html
QR+URL
- País de registro
- Precio medio en farmacia43.04 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BUPRENORFINA TEVA 70 MICROGRAMOS/HORA PARCHE TRANSDERMICO EFGForma farmacéutica: PARCHE TRANSDERMICO, 35 microgramos/hPrincipio activo: BuprenorfinaFabricante: Andromaco Pharma S.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 52,5 microgramos/hPrincipio activo: BuprenorfinaFabricante: Andromaco Pharma S.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 70 microgramos/hPrincipio activo: BuprenorfinaFabricante: Andromaco Pharma S.L.Requiere receta
Médicos online para BUPRENORFINA TEVA 70 MICROGRAMOS/HORA PARCHE TRANSDERMICO EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BUPRENORFINA TEVA 70 MICROGRAMOS/HORA PARCHE TRANSDERMICO EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes