BRIUMVI 150 MG CONCENTRADO PARA SOLUCION PARA PERFUSION

Cómo usar BRIUMVI 150 MG CONCENTRADO PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
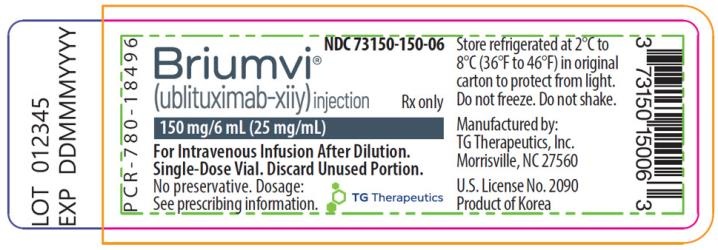
Briumvi 150 mg concentrado para solución para perfusión
ublituximab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a tenerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Briumvi y para qué se utiliza
- Qué necesita saber antes de empezar a recibir Briumvi
- Cómo se administra Briumvi
- Posibles efectos adversos
- Conservación de Briumvi
- Contenido del envase e información adicional
1. Qué es Briumvi y para qué se utiliza
Qué es Briumvi
Briumvi contiene el principio activo ublituximab. Es un tipo de proteína que recibe el nombre de anticuerpo monoclonal. Los anticuerpos actúan uniéndose a dianas específicas del cuerpo.
Para qué se utiliza Briumvi
Briumvi se utiliza para tratar a adultos con formas recurrentes de esclerosis múltiple (EMR), en las que el paciente tiene brotes (recidivas) seguidos de periodos con síntomas más suaves o sin síntomas.
Qué es la esclerosis múltiple
La esclerosis múltiple (EM) afecta al sistema nervioso central, especialmente a los nervios del encéfalo y de la médula espinal. En la EM, unos glóbulos blancos denominados linfocitos B, que forman parte del sistema inmunitario (el sistema de defensa del cuerpo), actúan incorrectamente y atacan una capa protectora (denominada vaina de mielina) que rodea las células nerviosas, causando así inflamación y lesión. La degradación de la vaina de mielina impide que los nervios funcionen correctamente y causa los síntomas de la EM. Los síntomas de la EM dependen de la parte del sistema nervioso central que esté afectada y pueden incluir problemas al caminar y de equilibrio, debilidad muscular, entumecimiento, visión doble y borrosa, problemas de coordinación y problemas de vejiga.
En las formas recurrentes de la EM, el paciente tiene crisis repetidas de síntomas (recidivas) que pueden aparecer súbitamente en un plazo de horas o lentamente a lo largo de varios días. Los síntomas desaparecen o mejoran entre las recidivas, pero la lesión puede acumularse y dar lugar a una discapacidad permanente.
¿Cómo actúa Briumvi?
Briumvi actúa uniéndose a una diana denominada CD20 en la superficie de los linfocitos B. Los linfocitos B son un tipo de glóbulo blanco que forma parte del sistema inmunitario. En la esclerosis múltiple, el sistema inmunitario ataca la capa protectora que rodea las células nerviosas. En este proceso intervienen los linfocitos B. Briumvi actúa sobre los linfocitos B y los elimina y, de esta forma, reduce la probabilidad de recidiva, alivia los síntomas y ralentiza la progresión de la enfermedad.
2. Qué necesita saber antes de empezar a recibir Briumvi
No debe recibir Briumvi:
- si es alérgicoal ublituximab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si tiene una infección grave;
- si le han dicho que tiene problemas graves del sistema inmunitario; o
- si tiene cáncer.
Si no está seguro, consulte a su médico antes de empezar a recibir Briumvi.
Advertencias y precauciones
Informe a su médico antes de empezar a recibir Briumvisi cumple alguno de los criterios indicados a continuación. Es posible que su médico decida posponer su tratamiento con Briumvi o que usted no puede recibir Briumvi si:
- tiene una infección. Su médico esperará a que se resuelva la infección antes de administrarle Briumvi.
- ha tenido alguna vez hepatitis Bo es portador del virus de la hepatitis B. Esto se debe a que medicamentos como Briumvi pueden activar de nuevo el virus de la hepatitis B. Antes del tratamiento con Briumvi, su médico comprobará si usted tiene riesgo de infección por el virus de la hepatitis B. Los pacientes que han tenido hepatitis B o que son portadores del virus de la hepatitis B se someterán a un análisis de sangre y serán objeto de vigilancia por un médico por si aparecen signos de infección por el virus de la hepatitis B.
- ha recibido recientemente alguna vacuna o podría recibir una vacuna en un futuro cercano.
- tiene cáncero ha tenido cáncer en el pasado. Es posible que su médico decida posponer el tratamiento.
Reacciones relacionadas con la perfusión
- El efecto adverso más frecuente del tratamiento con Briumvi son las reacciones relacionadas con la perfusión, un tipo de reacciones alérgicas que se producen durante o poco después de la administración de un medicamento. Pueden ser graves.
- Los síntomas de una reacción relacionada con la perfusión pueden ser, entre otros, los siguientes:
- picor en la piel
- urticaria
- enrojecimiento de la cara o de la piel
- irritación de la garganta
- problemas para respirar
- hinchazón de la lengua o de la garganta
- “pitos” al respirar (sibilancias)
- escalofríos
- fiebre
- dolor de cabeza
- mareo
- sensación de desmayo
- náuseas
- dolor abdominal (en el vientre)
- latido cardíaco rápido
- Informe inmediatamente a su médico o enfermero si tiene o cree que puede tener una reacción relacionada con la perfusión. Las reacciones relacionadas con la perfusión se pueden producir durante la perfusión o hasta 24 horas después de esta.
- Para reducir el riesgo de reacción relacionada con la perfusión, su médico le administrará otros medicamentos antes de cada perfusión de Briumvi (ver sección 3) y usted será objeto de una vigilancia estrecha durante la perfusión.
- Si sufre una reacción a la perfusión, es posible que su médico tenga que interrumpir o ralentizar la velocidad de la perfusión.
Infecciones
- Informe a su médico antes de recibir Briumvi si tiene o cree que tiene una infección. Su médico esperará a que se resuelva la infección antes de administrarle Briumvi.
- Podría contraer infecciones con mayor facilidad con Briumvi. Esto se debe a que las células inmunitarias sobre las que actúa Briumvi también ayudan a combatir las infecciones.
- Informe inmediatamente a su médico o enfermero si tiene una infección o alguno de los siguientes signos de infección durante o después del tratamiento con Briumvi:
- fiebre o escalofríos
- tos que no desaparece
- herpes (por ejemplo, calenturas [herpes labial], zóster o úlceras genitales)
- Informe inmediatamente a su médico o enfermero si cree que la EM está empeorando o nota algún síntoma nuevo.Esto se debe a una infección cerebral muy rara y potencialmente mortal denominada leucoencefalopatía multifocal progresiva (LMP), que puede causar síntomas similares a los de la EM. La LMP puede ocurrir en pacientes tratados con medicamentos como Briumvi y otros medicamentos para el tratamiento de la EM.
- Informe a su pareja o cuidadorsobre el tratamiento con Briumvi. Podrían notar síntomas de la LMP que usted no note, como lapsus de memoria, problemas de pensamiento, dificultad para caminar, pérdida de visión o cambios en la forma de hablar, que su médico podría tener que investigar.
Vacunas
- Informe a su médico si ha recibido recientemente alguna vacuna o podría recibir una vacuna en un futuro cercano.
- Su médico comprobará si necesita alguna vacuna antes de empezar el tratamiento con Briumvi. Debe recibir un tipo de vacuna llamado vacunas con microorganismos vivos o con microorganismos vivos atenuados al menos 4 semanas antes de empezar el tratamiento con Briumvi. Durante el tratamiento con Briumvi, no debe recibir vacunas con microorganismos vivos o con microorganismos vivos atenuados hasta que su médico le indique que su sistema inmunitario ya no está débil.
- Siempre que sea posible, debe recibir otros tipos de vacuna llamados vacunas inactivadas al menos 2 semanas antes de empezar el tratamiento con Briumvi. Si desea recibir vacunas inactivadas durante el tratamiento con Briumvi, consulte a su médico.
Niños y adolescentes
El uso de Briumvi no está indicado en niños y adolescentes menores de 18 años. Esto se debe a que todavía no se ha estudiado en este grupo de edad.
Otros medicamentos y Briumvi
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. En particular, informe a su médico:
- si está tomando, ha tomado recientemente o pudiera tener que tomar medicamentos que afectan al sistema inmunitario, como quimioterapia, inmunosupresores (excepto corticosteroides) u otros medicamentos que se utilizan para tratar la EM. Esto se debe a que pueden tener un efecto sumativo sobre el sistema inmunitario.
- si tiene previsto recibir alguna vacuna (ver “Advertencias y precauciones” más arriba).
Si cumple alguno de estos criterios (o no está seguro), consulte a su médico antes de empezar a recibir Briumvi.
Embarazo y lactancia
- Informe a su médico antes de empezar a recibir Briumvi si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada. Esto se debe a que Briumvi puede atravesar la placenta y afectar al feto.
- No utilice Briumvi si está embarazada a menos que lo haya comentado con su médico. Su médico sopesará el beneficio para usted de tomar Briumvi frente al riesgo para el feto.
- Si tiene un hijo y ha recibido Briumvi durante el embarazo, es importante que comunique al médico de su hijo que usted ha recibido Briumvi, para que el médico pueda recomendar cuándo debe ser vacunado su hijo.
- Se desconoce si Briumvi pasa a la leche materna. Consulte a su médico la mejor forma de alimentar a su hijo si usted recibe Briumvi.
Anticoncepción femenina
Si puede quedarse embarazada (concebir), debe utilizar métodos anticonceptivos:
- durante el tratamiento con Briumvi y
- durante al menos 4 meses después de la última perfusión de Briumvi.
Conducción y uso de máquinas
Es poco probable que Briumvi afecte a su capacidad para conducir o utilizar máquinas.
Briumvi contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Briumvi
Un médico o un enfermero con experiencia en el uso de este tratamiento le administrarán Briumvi. Le vigilará estrechamente mientras le estén administrando este medicamento. Esto es por si sufre algún efecto adverso. Siempre recibirá Briumvi con un gotero (perfusión intravenosa).
Medicamentos que se le administrarán antes de recibir Briumvi
Antes de recibir Briumvi, se le administrarán otros medicamentos para prevenir o reducir posibles efectos adversos tales como reacciones relacionadas con la perfusión (ver las secciones 2 y 4 para obtener información sobre las reacciones relacionadas con la perfusión).
Recibirá un corticosteroide y un antihistamínico antes de cada perfusión y es posible que reciba también otros medicamentos para reducir la fiebre.
Qué cantidad de Briumvi recibirá y con qué frecuencia la recibirá
- La primera dosis de Briumvi será de 150 mg. Esta perfusión durará 4 horas.
- La segunda dosis de Briumvi será de 450 mg administrados 2 semanas después de la primera dosis. Esta perfusión durará 1 hora.
- Las dosis posteriores de Briumvi serán de 450 mg administrados 24 semanas después de la primera dosis y cada 24 semanas posteriormente. Estas perfusiones durarán 1 hora.
Cómo se administra Briumvi
- Un médico o un enfermero le administrarán Briumvi. Briumvi debe diluirse antes de su administración. Un profesional sanitario realizará la dilución. Se administrará en forma de perfusión en una vena (perfusión intravenosa).
- Será objeto de una vigilancia estrecha durante la administración de Briumvi y durante al menos 1 hora después de la administración de las dos primeras perfusiones. Esto es por si sufre algún efecto adverso tal como una reacción relacionada con la perfusión. La perfusión se puede ralentizar, interrumpir temporalmente o interrumpir permanentemente si usted sufre una reacción relacionada con la perfusión, dependiendo de su gravedad (ver las secciones 2 y 4 para obtener información sobre las reacciones relacionadas con la perfusión).
Si olvidó una perfusión de Briumvi
- Si ha olvidado una perfusión de Briumvi, consulte a su médico para programarla lo antes posible. No espere a la siguiente perfusión programada.
- Para obtener el beneficio pleno de Briumvi, es importante que reciba cada perfusión en el momento oportuno.
Si interrumpe el tratamiento con Briumvi
- Es importante que continúe el tratamiento mientras usted y su médico decidan que está siendo beneficioso para usted.
- Algunos efectos adversos pueden estar relacionados con tener niveles bajos de linfocitos B. Después de interrumpir el tratamiento con Briumvi, es posible que experimente estos efectos adversos hasta que se restablezcan los niveles normales de linfocitos B.
- Antes de empezar a tomar otros medicamentos, indique a su médico cuándo recibió la última perfusión de Briumvi.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos con Briumvi:
Efectos adversos graves
Reacciones relacionadas con la perfusión
- Las reacciones relacionadas con la perfusión son el efecto adverso más frecuente del tratamiento con Briumvi (muy frecuentes: pueden afectar a más de 1 de cada 10 personas). En la mayoría de los casos son reacciones leves, pero pueden producirse algunas reacciones graves.
- Informe inmediatamente a su médico o enfermero si experimenta signos o síntomas de una reacción relacionada con la perfusión durante la perfusión o hasta 24 horas después de la perfusión.Los síntomas pueden ser, entre otros, los siguientes:
- picor en la piel
- urticaria
- enrojecimiento de la cara o de la piel
- irritación de la garganta
- problemas para respirar
- hinchazón de la lengua o de la garganta
- “pitos” al respirar (sibilancias)
- escalofríos
- fiebre
- dolor de cabeza
- mareo
- sensación de desmayo
- náuseas
- dolor abdominal (en el vientre)
- latido cardíaco rápido
- Si experimenta una reacción relacionada con la perfusión, le administrarán medicamentos para tratarla y es posible que sea necesario ralentizar o interrumpir la perfusión. Cuando la reacción haya cesado, se puede reanudar la perfusión. Si la reacción relacionada con la perfusión es potencialmente mortal, su médico interrumpirá permanentemente el tratamiento con Briumvi.
Infecciones
- Podría contraer infecciones con mayor facilidad con Briumvi. Algunas de ellas podrían ser graves. Se han observado las siguientes infecciones en pacientes tratados con Briumvi en la EM:
- Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- infecciones de las vías respiratorias altas (infecciones de nariz y garganta)
- infecciones de las vías respiratorias
- Frecuentes(pueden afectar a hasta 1 de cada 10 personas)
- infecciones de las vías respiratorias bajas (infecciones de los pulmones tales como bronquitis o neumonía)
- infecciones herpéticas (calenturas [herpes labial] o zóster)
- Informe inmediatamente a su médico o enfermero si nota alguno de los siguientes signos de infección:
- fiebre o escalofríos
- tos que no desaparece
- herpes (por ejemplo, calenturas [herpes labial], zóster o úlceras genitales)
Su médico esperará a que se resuelva la infección antes de administrarle Briumvi.
Otros efectos adversos
Frecuentes(pueden afectar a hasta 1 de cada 10 personas)
- neutropenia (nivel bajo de neutrófilos, un tipo de glóbulo blanco)
- dolor en una extremidad (brazos o piernas)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Briumvi
Conservar en nevera (entre 2 ºC y 8 ºC).
Los profesionales sanitarios conservarán Briumvi en el hospital o clínica en las siguientes condiciones:
- Este medicamento no se debe usar después de la fecha de caducidad que aparece en la caja y en la etiqueta del vial después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- Este medicamento se debe conservar en nevera (entre 2 ºC y 8 ºC). No se debe congelar. El vial se debe conservar en el embalaje exterior para protegerlo de la luz.
Se recomienda usar el producto inmediatamente después de la dilución. Si no se usa inmediatamente, los tiempos de conservación durante el uso y las condiciones antes del uso son responsabilidad del profesional sanitario y normalmente no deben exceder de 24 horas entre 2 ºC y 8 ºC y posteriormente 8 horas a temperatura ambiente.
Los medicamentos no se deben tirar por los desagües. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Briumvi
- El principio activo es ublituximab. Cada vial contiene 150 mg de ublituximab en 6 ml a una concentración de 25 mg/ml.
- Los demás componentes son cloruro de sodio, citrato de trisodio dihidrato, polisorbato 80, ácido clorhídrico y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- Briumvi es una solución entre transparente y opalescente y entre incolora y amarillenta.
- Se presenta en forma de concentrado para solución para perfusión.
- Este medicamento está disponible en envases que contienen 1 vial (vial de vidrio con 6 ml de concentrado).
Titular de la autorización de comercialización
Neuraxpharm Pharmaceuticals, S.L.
Avda. Barcelona 69
08970 Sant Joan Despí - Barcelona
España
Responsable de la fabricación
Millmount Healthcare
Block 7, City North Business Campus
Stamullen
Co. Meath
Irlanda
K32 YD60
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Neuraxpharm Belgium Tél/Tel: +32 (0)2 732 56 95 | Lietuva Neuraxpharm Pharmaceuticals, S.L. Tel:+34 93 475 96 00 |
Neuraxpharm Pharmaceuticals, S.L. Teπ.: +34 93 475 96 00 | Luxembourg/Luxemburg Neuraxpharm France Tél/Tel: +32 474 62 24 24 |
Ceská republika Neuraxpharm Bohemia s.r.o. Tel: +420 739 232 258 | Magyarország Neuraxpharm Hungary Kft. Tel.: +3630 464 6834 |
Danmark Neuraxpharm Sweden AB Tlf: +46 (0)8 30 91 41 (Sverige) | Malta Neuraxpharm Pharmaceuticals, S.L. Tel.:+34 93 475 96 00 |
Deutschland neuraxpharm Arzneimittel GmbH Tel: +49 2173 1060 0 | Nederland Neuraxpharm Netherlands B.V. Tel.: +31 70 208 5211 |
Eesti Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Norge Neuraxpharm Sweden AB Tlf:+46 (0)8 30 91 41 (Sverige) |
Ελλáδα Brain Therapeutics PC Τηλ: +302109931458 | Österreich Neuraxpharm Austria GmbH Tel.:+ 43 (0) 2236 320038 |
España Neuraxpharm Spain, S.L.U. Tel: +34 93 475 96 00 | Polska Neuraxpharm Polska Sp. z.o.o. Tel.: +48 783 423 453 |
France Neuraxpharm France Tél: +33 1.53.62.42.90 | Portugal Neuraxpharm Portugal, Unipessoal Lda Tel: +351 910 259 536 |
Hrvatska Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | România Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Ireland Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 | Slovenija Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Ísland Neuraxpharm Sweden AB Sími: +46 (0)8 30 91 41 (Svíþjóð) | Slovenská republika Neuraxpharm Slovakia a.s. Tel: +421 255 425 562 |
Italia Neuraxpharm Italy S.p.A. Tel: +39 0736 980619 | Suomi/Finland Neuraxpharm Sweden AB Puh/Tel: +46 (0)8 30 91 41 (Ruotsi/Sverige) |
Κúπρος Brain Therapeutics PC Τηλ: +302109931458 | Sverige Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 |
Latvija Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | United Kingdom (Northern Ireland) Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Esta información está destinada únicamente a profesionales sanitarios:
Leer la ficha técnica o resumen de las características del producto para obtener más información.
Posología
- Primera y segunda dosis
La primera dosis se administra en forma de perfusión intravenosa de 150 mg (primera perfusión), seguida de una perfusión intravenosa de 450 mg, 2 semanas después (segunda perfusión).
- Dosis posteriores
Las dosis posteriores de Briumvi se administran en forma de perfusión intravenosa única de 450 mg cada 24 semanas (tabla 1). La primera dosis posterior de 450 mg se debe administrar 24 semanas después de la primera perfusión. Se debe mantener un intervalo mínimo de 5 meses entre cada dosis de Briumvi.
Figura 1: Dosis y calendario de Briumvi
Primera perfusión | Segunda perfusión | Perfusiones anteriores |
Día 1
| Día 15
| Cada 6 meses
|
Tratamiento de las RRP antes de la perfusión
- El tratamiento debe ser iniciado y supervisado por un profesional sanitario con experiencia que tenga acceso a un apoyo médico adecuado para tratar reacciones graves tales como reacciones relacionadas con la perfusión (RRP) graves.
- Premedicación para las RRP
Se deben administrar como premedicación los dos medicamentos siguientes antes de cada perfusión de Briumvi para reducir la frecuencia y la gravedad de las RRP:
- 100 mg de metilprednisolona o 10-20 mg de dexametasona (o equivalente) aproximadamente 30-60 minutos antes de cada perfusión de Briumvi;
- difenhidramina aproximadamente 30-60 minutos antes de cada perfusión de Briumvi.
Además, se puede considerar también la premedicación con un antipirético (p. ej., paracetamol).
Instrucciones para la dilución
- Briumvi debe ser preparado por un profesional sanitario utilizando una técnica aséptica. No agitar el vial.
- El producto está previsto para un solo uso.
- No utilizar la solución si presenta un cambio de coloración o contiene partículas sólidas extrañas.
- El medicamento Briumvi se debe diluir antes de la administración. Las soluciones de Briumvi para administración intravenosa se preparan mediante dilución del producto en una bolsa de perfusión que contiene cloruro de sodio isotónico al 0,9 %. Para la primera perfusión, diluir un vial del producto en la bolsa de perfusión (150 mg/250 ml) hasta una concentración final de aproximadamente 0,6 mg/ml. Para las perfusiones posteriores, diluir tres viales del producto en la bolsa de perfusión (450 mg/250 ml) hasta una concentración final de aproximadamente 1,8 mg/ml.
- Antes de iniciar la perfusión intravenosa, el contenido de la bolsa de perfusión debe estar a temperatura ambiente.
Forma de administración
- Tras la dilución, Briumvi se administra en forma de perfusión intravenosa a través de una vía exclusiva.
- Las perfusiones de Briumvi no se deben administrar en forma de perfusión intravenosa lenta o en bolo.
Tabla 1: Dosis y calendario de Briumvi
Cantidad y volumen | Velocidad de perfusión | Duración 1 | |
Primera perfusión | 150 mg en 250 ml |
2 horas restantes. | 4 horas |
Segunda prefusión (2 semanas después) | 450 mg en 250 m |
30 minutos restantes. | 1 hora |
Perfusiones posteriores (una vez cada 24 semanas) | 450 mg en 250 m |
30 minutos restantes. | 1 hora |
1La duración de la perfusión puede ser mayor si la perfusión se interrumpe o ralentiza.
2La primera dosis posterior se debe administrar 24 semanas después de la primera perfusión.
Tratamiento de las RRP durante y después de la perfusión
Se debe vigilar a los pacientes durante la perfusión y durante al menos una hora después de la finalización de las dos primeras perfusiones.
Durante la perfusión
- Ajustes de la perfusión en caso de RRP
En caso de RRP durante una perfusión, se deben tener en cuenta los siguientes ajustes.
RRP potencialmente mortales
Si hay signos de una RRP potencialmente mortal o incapacitante durante una perfusión, se debe suspender inmediatamente la perfusión y se debe administrar al paciente el tratamiento adecuado. En estos pacientes se debe interrumpir de forma permanente el tratamiento con Briumvi (ver sección 4.3).
RRP graves
Si un paciente experimenta una RRP grave, se debe interrumpir inmediatamente la perfusión y se debe administrar al paciente tratamiento sintomático. La perfusión solo se debe reanudar una vez que hayan desaparecido todos los síntomas. Al reanudar la perfusión, empiece a la mitad de la velocidad de perfusión aplicada en el momento de la aparición de la RRP. Si la velocidad de perfusión se tolera, aumente la velocidad tal como se describe en la tabla 1.
RRP de leves a moderadas
Si un paciente experimenta una RRP de leve a moderada, se debe reducir la velocidad de perfusión a la mitad de la velocidad de perfusión aplicada en el momento de aparición del acontecimiento. Esta velocidad de perfusión reducida se debe mantener durante al menos 30 minutos. Si la velocidad de perfusión reducida se tolera, se puede aumentar la velocidad de perfusión tal como se describe en la tabla 1.
Después de la perfusión
- Se debe observar a los pacientes tratados con Briumvi durante al menos una hora después de la finalización de las dos primeras perfusiones por si aparecen síntomas de RRP.
- Los médicos deben informar a los pacientes de que se puede producir una RRP en las 24 horas siguientes a la perfusión.
Periodo de validez
Vial sin abrir
3 años
Solución para perfusión intravenosa diluida
- Se ha demostrado la estabilidad química y física durante el uso durante 24 horas entre 2 ºC y 8 ºC y posteriormente durante 8 horas a temperatura ambiente.
- Desde el punto de vista microbiológico, la solución para perfusión preparada debe usarse de inmediato. Si no se usa inmediatamente, los tiempos de conservación durante el uso y las condiciones antes del uso son responsabilidad del usuario y normalmente no deben exceder de 24 horas entre 2 ºC y 8 ºC y posteriormente 8 horas a temperatura ambiente, a menos que la dilución se haya realizado en condiciones asépticas controladas y validadas.
- Si no se puede finalizar una perfusión intravenosa en el mismo día, se debe desechar la solución restante.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BRIUMVI 150 MG CONCENTRADO PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para BRIUMVI 150 MG CONCENTRADO PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BRIUMVI 150 MG CONCENTRADO PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes