
BIMI 0,3 MG/ML COLIRIO EN SOLUCION

Cómo usar BIMI 0,3 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Bimi 0,3 mg/ml colirio en solución
Bimatoprost
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque
contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Bimi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bimi
- Cómo usar Bimi
- Posibles efectos adversos
- Conservación de Bimi
- Contenido del envase e información adicional
1. Qué es Bimi y para qué se utiliza
Bimi es un medicamento para el glaucoma. Pertenece a un grupo de medicamentos llamados prostamidas.
Este medicamento se utiliza para reducir la presión elevada del ojo. Este medicamento se puede usar solo o con otros colirios llamados betabloqueantes que también reducen la presión.
El ojo contiene un líquido transparente, acuoso, que mantiene la parte interior del ojo. Este líquido se drena continuamente fuera del ojo y se genera nuevo líquido para reemplazarlo. Si el líquido no se drena con la suficiente velocidad, aumenta la presión dentro del ojo. Este medicamento actúa aumentando el drenaje del líquido. Esto reduce la presión dentro del ojo. Si esta presión no se reduce, podría provocar una enfermedad denominada glaucoma y dañar su visión.
Este medicamento no contiene conservantes.
2. Qué necesita saber antes de empezar a usar Bimi
No use Bimi
- Si es alérgico al bimatoprost o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento, si:
- tiene algún problema respiratorio
- tiene problemas de hígado o riñones
- ha tenido una cirugía de catarata en el pasado
- presenta o ha presentado una tensión arterial baja o una frecuencia cardiaca baja
- ha padecido una infección viral o inflamación del ojo
- usa lentes de contacto (ver sección 3)
Durante el tratamiento, Bimi puede provocar una pérdida de grasa alrededor del ojo que puede causar profundización del surco palpebral, hundimiento de los ojos (enoftalmos), caída de los párpados superiores (ptosis), estiramiento de la piel alrededor del ojo (involución de la dermatocalasis) y que la parte blanca inferior del ojo se haga más visible (exposición escleral inferior). Los cambios suelen ser leves, pero si se acentúan, pueden afectar a su campo de visión. Los cambios pueden desaparecer si deja de usar Bimi.
Bimatoprost también puede causar oscurecimiento y crecimiento de las pestañas, así como oscurecimiento de la piel alrededor del párpado. Puede oscurecerse el color del iris. Estos cambios puede que sean permanentes y más visibles si sólo se está tratando un ojo.
Si tiene antecedentes de hipersensibilidad por contacto a la plata, no debe usar este medicamento.
Niños y adolescentes
Este medicamento no se ha estudiado en menores de 18 años y, por tanto, no debe utilizarse en pacientes de menos de 18 años de edad.
Otros medicamentos y Bimi
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Si está usando otras gotas para los ojos, deje al menos 5 minutos entre la aplicación de Bimi y las otras gotas. Las pomadas oftálmicas deben administrarse en último lugar.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Este medicamento puede pasar a la leche materna, por tanto, no debería utilizarlo si está en periodo de lactancia.
Conducción y uso de máquinas
Tras la instilación de bimatoprost, puede aparecer visión borrosa durante un corto periodo de tiempo. No conduzca ni use máquinas hasta ver con claridad.
Bimi contiene fosfatos
Este medicamento contiene 0,95 mg de fosfatos en cada mililitro. Si sufre de daño grave en la capa transparente de la parte frontal del ojo (la córnea), el tratamiento con fosfatos, en casos muy raros, puede provocar parches nublados en la córnea debido al calcio.
3. Cómo usar Bimi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Este medicamento solo debe aplicarse en los ojos. La dosis recomendada es una gota de bimatoprost una vez al día, por la noche, en cada ojo que necesite tratamiento.
Bimatoprost no se ha estudiado en pacientes que usan lentes de contacto. Las lentes de contacto deben quitarse antes de la instalación y pueden volver a colocarse 15 minutos después de la administración.
No lo use más de una vez al día ya que la efectividad del tratamiento puede verse reducida.
Bimi es una solución estéril que no contiene conservantes. Ver sección 6. Aspecto de Bimi y contenido del envase.
Antes de la instilación de las gotas para los ojos:
- Cuando lo use por primera vez, antes de administrar una gota en el ojo, primero debe practicar el uso del frasco gotero apretándolo lentamente para liberar una gota en el aire, lejos del ojo.
- Cuando esté seguro de que puede administrar una gota a la vez, debe elegir la posición que le resulte más cómoda para la instilación de las gotas (puede sentarse, acostarse boca arriba o pararse frente a un espejo).
Instrucciones de uso:
- Lávese las manos cuidadosamente antes de usar este medicamento.
- Si el envase o el frasco están dañados, no utilice el medicamento.
- Cuando use el medicamento por primera vez, desenrosque la tapa después de asegurarse de que el anillo sellado en la tapa no se haya roto. Debería sentir una ligera resistencia hasta que este anillo a prueba de manipulaciones se rompa (vea la imagen 1).
- Si el anillo a prueba de manipulaciones está suelto, deséchelo porque podría caerse en el ojo y causar lesiones.
- Incline la cabeza hacia atrás y tire suavemente hacia abajo del párpado inferior para formar una bolsa entre el ojo y el párpado (vea la imagen 2). Evite el contacto entre la punta del frasco y su ojo, párpados o dedos.
- Instile una gota en la bolsa presionando lentamente sobre el frasco (ver imagen 3). Apriete el frasco suavemente en el medio y deje caer una gota en su ojo. Puede haber un retraso de unos segundos entre la compresión y la salida de la gota. No apriete demasiado. Si no está seguro de cómo administrar su medicamento, consulte con su médico, farmacéutico o enfermero.
- Comprima el conducto lagrimal durante unos 2 minutos (presionando un dedo contra el rabillo del ojo junto a la nariz), y cierre los ojos y manténgalos cerrados durante este tiempo. Esto asegura que la gota sea absorbida por el ojo y que probablemente se reduzca la cantidad de medicamento que se drena a través del conducto lagrimal hacia la nariz.
- Repita los pasos 5 y 6 en el otro ojo si su médico le ha indicado que lo haga.
- Después de su uso y antes de volver a tapar, el frasco debe agitarse una vez hacia abajo, sin tocar la punta del gotero, para eliminar cualquier líquido residual en la punta. Esto es necesario para garantizar la entrega de gotas posteriores. Después de la instilación, enrosque el tapón del frasco (ver imagen 4).
- Información adicional para el frasco que contiene 9 ml de solución: Al final de los 90 días del periodo de validez del medicamento una vez abierto, quedará un poco de Bimi en el frasco. No intente utilizar el exceso de medicamento restante en el frasco. No utilice el colirio durante más de 90 días tras la primera apertura del frasco.
Si una gota no le alcanza el ojo, vuelva a intentarlo.
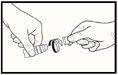

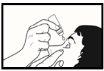
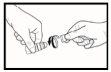
imagen 1imagen 2imagen 3imagen 4
Si usa másBimidel que debe
Si usa más cantidad de este medicamento del que debe, es improbable que ello le cause ningún daño serio. Aplique la siguiente dosis a la hora habitual. Si está preocupado/a, hable con su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, consulte a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usarBimi
Si olvidó aplicar este medicamento, use una sola gota tan pronto como se acuerde y vuelva después a su rutina habitual. No aplique una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento conBimi
Este medicamento debe usarse cada día para que funcione bien. Si deja de usarlo, la presión en el interior del ojo puede aumentar, así pues, consulte con su médico antes de interrumpir el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta algún efecto secundario, consulte a su médico o farmacéutico. Esto incluye los posibles efectos secundarios que no se mencionan en este prospecto.
Efectos adversos muy frecuentes
Éstos pueden afectar a más de 1 de cada 10 pacientes
Que afectan al ojo
- Ligero enrojecimiento (hasta el 24% de las personas)
- Pérdida de grasa en la región del ojo que puede causar profundización del surco palpebral, hundimiento de los ojos (enoftalmos), caída de los párpados (ptosis), estiramiento de la piel alrededor del ojo (involución de la dermatocalasis) y que la parte blanca inferior del ojo se haga más visible (exposición escleral inferior).
Efectos adversos frecuentes
Éstos pueden afectar hasta 1 y 9 de cada 100 pacientes
Que afectan al ojo
- Pequeñas erosiones en la superficie del ojo, con o sin inflamación
- Irritación
- Picor en los ojos
- Dolor
- Sequedad
- Sensación de tener algo en el ojo
- Pestañas más largas
- Piel de color más oscuro alrededor del ojo
- Párpados enrojecidos
Efectos adversos poco frecuentes
Éstos pueden afectar a entre 1 y 9 de cada 1.000 pacientes
Que afectan al ojo
- Ojos cansados
- Sensibilidad a la luz
- Iris más oscuro
- Párpados inflamados y con picor
- Lagrimeo
- Inflamación de la capa transparente que cubre la superficie del ojo
- Visión borrosa
Que afectan al cuerpo
- Dolores de cabeza
- Crecimiento de vello alrededor del ojo
Efectos adversos de frecuencia no conocida
Que afectan al ojo
- Ojos pegajosos
- Molestias oculares
Que afectan al cuerpo
- Asma
- Empeoramiento del asma
- Empeoramiento de la enfermedad pulmonar denominada enfermedad pulmonar obstructiva crónica (EPOC)
- Dificultad para respirar
- Síntomas de reacción alérgica (inflamación, enrojecimiento del ojo y erupción en la piel)
- Mareo
- Presión arterial elevada
- Decoloración de la piel (periocular)
Además de los efectos adversos de bimatoprost 0,3 mg/ml unidosis, se han observado los efectos adversos siguientes con la formulación multidosis con conservantes para bimatoprost 0,3 mg/ml y pueden producirse en pacientes que utilizan bimatoprost 0,3 mg/ml unidosis:
- Sensación de quemazón en el ojo
- Reacción alérgica en el ojo
- Inflamación del párpado
- Dificultad para ver claramente
- Empeoramiento de la visión
- Pestañas más oscuras
- Hemorragia retiniana
- Inflamación en el interior del ojo
- Edema macular cistoide (inflamación de la retina dentro del ojo que conduce al empeoramiento de la visión)
- Inflamación del iris
- Fasciculaciones del párpado
- El párpado se ha contraído y separado de la superficie del ojo
- Náuseas
- Enrojecimiento de la piel alrededor del ojo
- Debilidad
- Aumento en algunos de los valores de los análisis de sangre que indican cómo está funcionando el hígado
Otros efectos adversos notificados con colirios que contienen fosfatos:
Si sufre de daño grave en la capa transparente de la parte frontal del ojo (la córnea), el tratamiento con fosfatos, en casos muy raros, puede provocar parches nublados en la córnea debido al calcio.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bimi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el frasco después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere ninguna condición especial de conservación.
Después de abrir el frasco por primera vez, conservar durante 90 días a una temperatura inferior a 25 °C.
Desechar 90 días después de la primera apertura del frasco.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Bimi
- El principio activo es bimatoprost.
Cada ml de solución contiene 0,3 mg de bimatoprost.
Cada gota contiene aproximadamente 0,0087 mg de bimatoprost.
Cada frasco contiene 3 ml o 9 ml de solución.
- Los demás componentes son hidrogenofosfato de sodio dodecahidrato, ácido cítrico monohidratado, cloruro sódico, ácido clorhídrico diluido (para ajustar el pH), agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Bimi es una solución transparente e incolora.
Este medicamento está disponible en un frasco de LDPE blanco (5 ml o 11 ml) con un aplicador de gotero de HDPE multidosis, que evita la contaminación del contenido debido a un sistema de válvula de silicona, al retorno del aire de filtrado al frasco y al tapón de rosca de HDPE a prueba de manipulaciones, y la caja de cartón.
Tamaños de envase: cajas que contienen 1 o 3 frascos de 3 ml de solución o 1 frasco de 9 ml de solución.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
BRILL PHARMA, S.L.
C/ Munner, 8
08022 Barcelona
España
Responsable de la fabricación
Rafarm S.A.
Thesi Pousi Xatzi Agiou Louka
Paiania, 190 02
Grecia
Fecha de la última revisión de este prospecto:Julio 2022
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Precio medio en farmacia39.71 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BIMI 0,3 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Tiedra Farmaceutica S.L.Requiere recetaForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Sifi S.P.A.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/mlPrincipio activo: BimatoprostFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para BIMI 0,3 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BIMI 0,3 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















