
BELOKEN 1 mg/ml SOLUCION INYECTABLE

Cómo usar BELOKEN 1 mg/ml SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Beloken 1 mg/ml solución inyectable
metoprolol tartrato
Lea todo el prospecto detenidamente antesde que le administreneste medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Beloken 1 mg/ml para qué se utiliza
- Qué necesita saber antes de empezar a usar Beloken 1 mg/ml
- Cómo se le administrará Beloken 1 mg/ml
- Posibles efectos adversos
- Conservación de Beloken 1 mg/ml
Contenido del envase e información adicional
1. Qué es Beloken 1 mg/ml y para qué se utiliza
El principio activo es metoprolol tartrato, que pertenece a un grupo de medicamentos denominados betabloqueantes. Estos medicamentos reducen el efecto de las hormonas producidas por estrés sobre algunas partes del organismo. De este modo, metoprolol es capaz de actuar sobre los vasos sanguíneos y el corazón reduciendo la presión arterial y la frecuencia cardíaca.
Este medicamento se utiliza en adultos para el tratamiento de:
- Alteraciones del ritmo cardíaco, especialmente latidos cardíacos rápidos (taquicardia supraventricular).
- Tratamiento de pacientes hemodinámicamente estables con infarto agudo de miocardio definido o sospechado. (Intervención precoz en la fase aguda, previa a la terapia oral).
2. Qué necesita saber antes de usar Beloken 1 mg/ml
No use Beloken 1 mg/ml
- si es alérgico al metoprolol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si sufre de insuficiencia cardíaca no compensada, bloqueo (trastorno de la conducción cardíaca) o infarto agudo de miocardio.
- si ha presentado alguna vez latidos cardíacos muy lentos o muy irregulares o insuficiencia circulatoria.
El uso de Beloken 1 mg/ml no está recomendado en niños.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Beloken:
- si presenta signos/síntomas de niveles sanguíneos bajos de glucosa en sangre (hipoglucemia).
- si tiene problemas de salud tales como asma o dificultades respiratorias, trastornos circulatorios, problemas cardíacos, renales o de tiroides.
- si durante el tratamiento con este medicamento su ritmo cardíaco se enlentece más y más.
- si le han informado alguna vez que padece feocromocitoma (un tumor en las glándulas suprarrenales).
- si tiene la tensión arterial muy baja o la circulación sanguínea muy deteriorada.
- si padece insuficiencia cardíaca no controlada.
- si usted es alérgico a picaduras de insectos, alimentos u otras sustancias.
Informe a su médico sobre cualquier problema de salud que haya tenido en el pasado.
Si es deportista, tenga en cuenta que este medicamento contiene un componente que puede establecer un resultado analítico de control del dopaje como positivo.
Si no ha informado a su médico sobre alguna de las situaciones anteriores o tiene alguna duda, consulte con su médico o farmacéutico antes de comenzar el tratamiento con este medicamento.
Uso de Beloken 1 mg/ml con otros medicamentos
Comunique a su médico o farmacéutico si está tomando ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Algunos medicamentos pueden influir sobre la acción de otros medicamentos.
En especial informe a su médico si está utilizando alguno de los siguientes medicamentos, ya que puede que tenga que ajustar la dosis de alguno de los medicamentos:
- Medicamentos empleados en tratamientos cardíacos y de los vasos sanguíneos (tales como digitálicos/digoxina, antagonistas del calcio (verapamilo o diltiazem), agentes antiarrítmicos (quinidina o amiodarona), agentes bloqueantes de ganglios simpáticos, hidralazina, clonidina).
- Otros fármacos tales como los inhibidores de la monoaminooxidasa (IMAO), anestésicos por inhalación, antibióticos (rifampicina), fármacos antiulcerosos (cimetidina), antiinflamatorios no esteroideos (p. ej. indometacina, celecoxib), ciertos medicamentos antidepresivos y antipsicóticos, antihistamínicos, otros betabloqueantes (p. ej. colirios, medicamentos para el tratamiento del asma) y otras sustancias (p.ej. alcohol, algunas hormonas, como la adrenalina), antidiabéticos orales.
Tenga en consideración que:
- Si toma conjuntamente clonidina y Beloken 1 mg/ml y debe interrumpir el tratamiento con clonidina, deberá dejar de tomar metoprolol varios días antes que la clonidina.
- Si está tomando antidiabéticos orales, puede ser necesario que su médico necesite ajustar la dosis.
- Los niveles en sangre de este medicamento pueden verse aumentados si se administra junto con antiarrítmicos, antihistamínicos, antidepresivos, antipsicóticos, antiinflamatorios no esteroideos, alcohol o hidralazina.
- Los niveles en sangre de este medicamento pueden verse disminuidos por rifampicina.
- La administración de este medicamento junto con medicamentos antiarrítmicos (quinidina o amiodarona) produce un aumento del efecto de estos medicamentos.
- Los efectos de este medicamento pueden verse aumentados cuando se administra junto con antagonistas del calcio (verapamilo o diltiazem). Debe evitarse la administración conjunta de estos medicamentos.
- Los efectos de este medicamento pueden verse disminuidos cuando se administra junto con adrenalina o indometacina.
Toma de Beloken 1 mg/ml con alcohol
La ingesta de alcohol puede aumentar los niveles de metoprolol en sangre, incrementándose el efecto del medicamento. Evite tomar alcohol junto con este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de que le administren este medicamento.
Los betabloqueantes, incluyendo el metoprolol, pueden dañar al feto y provocar el parto prematuro.
Comunique a su médico si está en periodo de lactancia, puesto que Beloken 1 mg/ml puede causar efectos adversos en el lactante, como enlentecimiento del ritmo cardíaco.
Conducción y uso de máquinas
Algunos pacientes pueden sentirse cansados o mareados cuando toman Beloken. Si esto le ocurre, no conduzca ni maneje herramientas o máquinas.
Beloken 1 mg/ml contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por 5 ml de solución inyectable (una ampolla); esto es, esencialmente “exento de sodio”.
3. Cómo se le administrará Beloken 1 mg/ml
Metoprolol inyección intravenosa se emplea en situaciones agudas en las que sea necesaria una rápida aparición de la acción y sólo debe ser administrado por personal experto que puede dar más información.
Su médico le indicará la duración de su tratamiento con este medicamento. No suspenda el tratamiento a menos que su médico se lo diga.
Tratamiento de pacientes hemodinámicamente estables con infarto agudo de miocardio definido o sospechado. (Intervención precoz en la fase aguda, previa a la terapia oral):En la fase aguda y tan pronto como sea posible tras la llegada del paciente al hospital, se administrarán 5 mg de metoprolol intravenosamente en forma de bolo. Con intervalo de 2 minutos se repetirá una segunda y una tercera inyección de 5 mg, dependiendo del estado hemodinámico del paciente. (Véase “Antes de usar Beloken 1 mg/ml).
A los pacientes que hayan tolerado la dosis total intravenosa de 15 mg se les administrará, transcurridos 15 minutos de la última inyección, comprimidos de 50 mg de metoprolol succinato o tartrato 4 veces al día, durante 48 horas.
Los pacientes que no toleren la dosis total intravenosa de 15 mg, el tratamiento oral se iniciará con precaución empezando con una dosis más baja.
Alteraciones del ritmo cardíaco, en particular especialmente latidos cardíacos rápidos (taquicardia supraventricular):Inicialmente hasta 5 mg administrados intravenosamente a razón de 1-2 mg/minuto. Esta dosis puede ser repetida con 5 minutos de intervalo hasta alcanzar el efecto satisfactorio. La dosis total de 10-15 mg consigue generalmente este efecto.
Dosis de 20 mg o más son innecesarias, ya que no consiguen un mayor beneficio terapéutico.
Uso en niños y adolescentes
No está recomendado debido a la poca experiencia con Beloken en niños y adolescentes.
Uso en pacientes de edadavanzada
La dosis de este medicamento no necesita ser ajustada en pacientes de edad avanzada.
Si estima que la acción de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico, personal sanitario o farmacéutico.
Si se le administra másBeloken 1 mg/ml del que debe
Si considera que le han administrado demasiado Beloken 1mg/ml, consulte con su médico o farmacéutico inmediatamente.
En caso de utilizar una dosis de este medicamento superior a la recomendada, si ésta es suficientemente alta, puede sufrir una intoxicación con alguno de los siguientes síntomas: ritmo cardíaco lento o irregular, dificultad al respirar, hinchazón de tobillos, sensación de palpitaciones, desvanecimiento, mareos, dolor torácico, piel fría, pulso débil, confusión mental, ansiedad, paro cardíaco, pérdida total o parcial del conocimiento (o incluso coma), náuseas, vómitos, coloración azul de la piel o hipotensión (tensión arterial baja).
Las primeras manifestaciones de la sobredosis pueden observarse entre 20 minutos y 2 horas después de la administración del fármaco. En caso de presentar alguno de estos síntomas, contacte inmediatamente con su médico, farmacéutico u hospital más próximo.
En caso de tomar alcohol, fármacos antihipertensivos, quinidina o somníferos (barbitúricos) junto con metoprolol, pueden agravarse los síntomas.
Tratamiento:
En caso de hipotensión grave y shock se administrará plasma o sustitutos del plasma y se le mantendrá en observación en la unidad de cuidados intensivos.
Si el ritmo cardíaco es excesivamente lento o irregular, puede utilizarse atropina vía intravenosa y/o un marcapasos. Si fuera necesario, también pueden administrarse glucagón y/o dobutamina y puede considerarse la administración de iones de calcio.
El broncoespasmo puede revertirse con un broncodilatador. Para mayor información ver Ficha Técnica.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Beloken 1 mg/ml es bien tolerado y los efectos indeseados que puedan ocurrir son, generalmente, leves y desaparecen al interrumpir el tratamiento.
Se han comunicado los siguientes efectos adversos en pacientes tratados con metoprolol, aunque la relación con el tratamiento con metoprolol no ha sido establecida en todos los casos. Si experimenta alguna de las reacciones adversas descritas a continuación de forma persistente, comuníquelo a su médico.
Muy frecuentes(puedenafectar a más de 1 de cada 10 personas):
- cansancio.
Frecuentes(puedenafectar hasta 1 de cada 10 personas):
- mareos, dolor de cabeza,
- enlentecimiento del ritmo cardíaco, mareos al cambiar de posición (muy raramente con pérdida del conocimiento), manos y pies fríos, palpitaciones,
- náuseas, dolor abdominal, diarrea, estreñimiento,
- sensación de ahogo al realizar un esfuerzo.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- sensación de acaloramiento/pinchazos/entumecimiento, calambres musculares,
- síntomas de enfermedad cardíaca tales como ahogo, decaimiento e hinchazón de los tobillos pueden empeorar temporalmente,
- durante un ataque cardíaco la presión arterial puede disminuir excesivamente (shock cardiogénico), alteraciones menores del electrocardiograma sin que resulte afectada la función cardíaca, hinchazón, dolor torácico,
- depresión, alteración de la concentración, somnolencia, falta de sueño, pesadillas,
- erupción cutánea, aumento de la sudoración,
- sensación de opresión en las vías respiratorias,
- vómitos,
- aumento de peso.
Raros (pueden afectar hasta 1 de cada 1.000 personas):
- alteraciones de la conducción cardíaca en el electrocardiograma, latidos cardíacos irregulares,
- nerviosismo, ansiedad,
- problemas hepáticos (anomalías en las pruebas de función hepática),
- pérdida de cabello,
- goteo nasal debido a reacción alérgica,
- trastornos de visión, sequedad y/o irritación de ojos,
- sequedad de boca, lagrimeo o irritación de ojos debido a reacción alérgica,
- impotencia/disfunción sexual.
Muy raros(puedenafectar hasta 1 de cada 10.000 personas):
- empeoramiento de los problemas circulatorios de las extremidades en pacientes con trastornos circulatorios graves,
- dolor en las articulaciones,
- pérdida o deterioro de la memoria, confusión, alucinaciones,
- reacción cutánea debido a un aumento de la sensibilidad al sol, empeoramiento de la psoriasis,
- zumbido de oídos,
- alteraciones del gusto,
- alteraciones sanguíneas (disminución del número de plaquetas en sangre)
- hepatitis.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Beloken 1 mg/ml
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC.
Conservar las ampollas en el envase original hasta el momento de utilizarlas.
6. Contenido del envase e información adicional
Composición de Beloken 1 mg/ml
- El principio activo es metoprolol tartrato 5 mg.
- Los demás componentes (excipientes) son: cloruro de sodio, agua para inyección, c.s.p. 5 ml.
Aspecto del producto y contenido del envase
Beloken 1 mg/ml se presenta en un envase de 5 ampollas de vidrio de 5 mg (1 mg/ml).
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali, 1
20148 Milano
Italia
Responsable de la fabricación:
Cenexi
52 Rue Marcel et Jacques Gaucher
94120 Fontenay Sous Bois
Francia
o
CIT S.r.l.
Via Primo Villa, 17
20875 Burago di Molgora (MB)
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Casen Recordati, S.L.
Autovía de Logroño, km 13,300
50180 Utebo - Zaragoza
España
Fecha de la última revisión de este prospecto: enero 2021
Esta información está destinada únicamente a médicos o profesionales del sector sanitario:
MODO DE EMPLEO
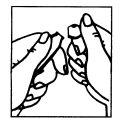
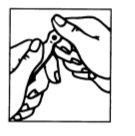
Figura 1 Figura 2
- La ampolla presenta una marca debajo del punto azul (Figura 1).
- Poner el pulgar en el punto azul y romper la ampolla por el cuello (Figura 2).
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BELOKEN 1 mg/ml SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: metoprololFabricante: Casen Recordati S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: metoprololFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: AtenololFabricante: Laboratorios Alter S.A.Requiere receta
Médicos online para BELOKEN 1 mg/ml SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BELOKEN 1 mg/ml SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











