
BECLOMETASONA/FORMOTEROL CIPLA 100 MICROGRAMOS/6 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION

Cómo usar BECLOMETASONA/FORMOTEROL CIPLA 100 MICROGRAMOS/6 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Beclometasona/Formoterol Cipla y para qué se utiliza
- Qué necesita saber antes de empezar a usar Beclometasona/Formoterol Cipla
- Cómo usar Beclometasona/Formoterol Cipla
- Posibles efectos adversos
- Conservación de Beclometasona/Formoterol Cipla
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Beclometasona/Formoterol Cipla 100 microgramos/6 microgramos/pulsación, solución para inhalación en envase a presión.
dipropionato de beclometasona/fumarato de formoterol dihidrato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Beclometasona/Formoterol Cipla y para qué se utiliza
- Qué necesita saber antes de empezar a usar Beclometasona/Formoterol Cipla
- Cómo usar Beclometasona/Formoterol Cipla
- Posibles efectos adversos
- Conservación de Beclometasona/Formoterol Cipla
- Contenido del envase e información adicional
1. Qué es Beclometasona/Formoterol Cipla y para qué se utiliza
Beclometasona/Formoterol Cipla es una solución para inhalación en envase a presión que contiene dos principios activos que se inhalan por la boca y se liberan directamente en los pulmones.
Los dos principios activos son dipropionato de beclometasona y fumarato de formoterol dihidrato. El dipropionato de beclometasona pertenece a un grupo de medicamentos denominados corticosteroides que poseen una acción antiinflamatoria que reduce la inflamación e irritación de sus pulmones.
El fumarato de formoterol dihidrato pertenece a un grupo de medicamentos denominados broncodilatadores de acción prolongada que relajan los músculos de las vías respiratorias por lo que le ayudan a respirar más fácilmente.
Estos dos principios activos combinados facilitan la respiración, ya que proporcionan alivio de síntomas como la dificultad para respirar, las sibilancias y la tos en pacientes asmáticos o con EPOC, y también ayudan a prevenir los síntomas del asma.
Asma
Este medicamento está indicado en el tratamiento habitual del asma en pacientes adultos en quienes:
- El asma está insuficientemente controlada mediante el uso de corticosteroides inhalados y broncodilatadores de acción corta administrados "a demanda".
o:
- El asma está respondiendo bien a un tratamiento combinado de corticosteroides y broncodilatadores de acción prolongada.
EPOC
Este medicamento puede también usarse para tratar los síntomas de la enfermedad pulmonar obstructiva crónica (EPOC) grave en pacientes adultos. EPOC es una enfermedad crónica de las vías respiratorias pulmonares causada principalmente por fumar cigarrillos.
2. Qué necesita saber antes de empezar a usar Beclometasona/Formoterol Cipla
No use Beclometasona/Formoterol Cipla:
- Si es alérgico a dipropionato de beclometasona o fumarato de formoterol dihidrato o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, o farmacéutico o enfermero antes de empezar a usar
Beclometasona/Formoterol Cipla:
- Si presenta problemas cardíacos, como una angina de pecho (dolor cardíaco, dolor en el pecho), un ataque al corazón reciente (infarto de miocardio), una insuficiencia cardíaca, un estrechamiento de las arterias que rodean el corazón (cardiopatía coronaria), una enfermedad de las válvulas del corazón o cualquier otra anomalía conocida del corazón, o si padece una enfermedad denominada cardiomiopatía hipertrófica obstructiva (también llamada CMH, en la que el músculo cardíaco está agrandado).
- Si padece un estrechamiento de las arterias (también denominado arterioesclerosis), si tiene hipertensión arterial o si sabe que tiene un aneurisma (un abombamiento anormal de la pared vascular).
- Si padece trastornos del ritmo cardíaco, como aumento de la frecuencia cardíaca o frecuencia cardíaca irregular, tiene el pulso acelerado o sufre palpitaciones, o se le ha informado de que presenta alteraciones electrocardiográficas.
- Si su glándula tiroides es hiperactiva.
- Si su concentración de potasio en sangre es baja.
- Si padece cualquier enfermedad hepática o renal.
- Si es diabético (la inhalación de dosis elevadas de formoterol puede provocar un aumento de la concentración de glucosa en sangre y, por lo tanto, deberá someterse a análisis de sangre adicionales para controlar el azúcar en sangre cuando comience a utilizar el inhalador y de vez en cuando durante el tratamiento).
- Si tiene un tumor de las glándulas suprarrenales (denominado feocromocitoma).
- Si va a recibir anestesia. En función del agente anestésico, puede que deba suspender el uso de este medicamento un mínimo de 12 horas antes de la anestesia.
- Si va a ser tratado o ya ha sido tratado de tuberculosis (TB) o si padece alguna infección vírica o fúngica en pulmón conocida.
- Si no puede tomar alcohol por cualquier razón.
Si alguno de los supuestos anteriores es aplicable en su caso, informe siempre a su médico antes de usar beclometasona/formoterol.
Si tiene o ha tenido problemas médicos o alergias o si no está seguro de si puede usar este medicamento, consulte a su médico, enfermero o farmacéutico antes de usar este medicamento.
El tratamiento con un agonista β2 como el formoterol contenido en este medicamento puede ocasionar una caída brusca de la concentración de potasio en suero (hipopotasemia).
Tenga especial cuidado si padece asma grave.La razón es que una falta de oxígeno en la sangre y otros tratamientos que puede estar recibiendo junto con beclometasona/formoterol, como medicamentos para tratar las enfermedades cardíacas o la tensión arterial alta (conocidos como diuréticos) u otros medicamentos usados para tratar el asma, pueden empeorar la caída de las concentraciones de potasio. Por ello, su médico querrá comprobar su concentración de potasio en sangre de vez en cuando.
Si toma dosis más altas de corticosteroides inhalados durante períodos prolongados, puede que precise de corticosteroides en situaciones de estrés. Este tipo de situaciones incluyen el ingreso hospitalario tras un accidente, la existencia de una lesión grave o antes de una intervención quirúrgica. En tal caso, el médico que le trata decidirá si es necesario aumentar la dosis de corticoesteroides y puede que le prescriba comprimidos o inyecciones de esteroides.
En caso de acudir al hospital, recuerde llevar consigo todos los medicamentos e inhaladores, incluidos beclometasona/formoterol y los demás medicamentos o comprimidos adquiridos sin receta, a ser posible dentro de su envase original.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Niños y adolescentes
Este medicamento no debe usarse en niños ni adolescentes menores de 18 años, hasta que no se disponga de más datos.
Otros medicamentos y Beclometasona/Formoterol Cipla
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar otros medicamentos, incluidos los medicamentos para el asma y la EPOC, o otros medicamentos adquiridos sin receta.
Algunos medicamentos pueden aumentar los efectos de beclometasona/formoterol, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
No utilice betabloqueantes con este medicamento
Los betabloqueantes como atenolol, propranolol y sotalol se usan para tratar una serie de afecciones, incluida la presión arterial alta y afecciones cardíacas, como ritmos cardíacos anormales e insuficiencia cardíaca; el timolol se usa para tratar el glaucoma. Si necesita utilizar betabloqueantes, incluidos los colirios, es probable que el efecto del formoterol disminuya o incluso sea nulo. En cambio, el uso de otros fármacos beta adrenérgicos (fármacos que funcionan del mismo modo que el formoterol) puede potenciar los efectos del formoterol.
El uso combinado de Beclometasona/Formoterol Cipla con:
- Medicamentos para tratar trastornos del ritmo cardíaco (quinidina, disopiramida, procainamida), medicamentos para tratar reacciones alérgicas (antihistamínicos), medicamentos para tratar síntomas de la depresión o trastornos psíquicos, como inhibidores de la monoaminooxidasa (por ejemplo, fenelzina e isocarboxazida), antidepresivos tricíclicos (por ejemplo, amitriptilina e imipramina) y fenotiazinas, pueden dar lugar a alteraciones electrocardiográficas (ECG, trazado electrocardiográfico). También pueden aumentar el riesgo de trastornos del ritmo cardíaco (arritmias ventriculares).
- Medicamentos para tratar la enfermedad de Parkinson (L-dopa) o para tratar una glándula tiroides hipoactiva (L-tiroxina), medicamentos que contienen oxitocina (que provoca la contracción del útero) y alcohol, pueden reducir la tolerancia cardíaca a los agonistas β2, como el formoterol.
- Inhibidores de la monoaminooxidasa (IMAO), incluidos fármacos con propiedades parecidas, como furazolidona y procarbazina, usados para tratar trastornos psíquicos, pueden originar un aumento de la tensión arterial.
- Medicamentos para tratar enfermedades cardíacas (digoxina), pueden ocasionar una caída de la concentración de potasio en suero. Esto puede aumentar las probabilidades de sufrir trastornos del ritmo cardíaco.
- Otros medicamentos utilizados para tratar el asma (teofilina, aminofilina o esteroides) y diuréticos, pueden producir una caída de la concentración de potasio en suero.
- Algunos anestésicos pueden incrementar el riesgo de sufrir trastornos del ritmo cardíaco.
Embarazo, lactancia y fertilidad
No se dispone de datos clínicos sobre el uso de beclometasona/formoterol durante el embarazo.
No use beclometasona/formoterol si está usted embarazada o cree que puede estarlo, si tiene intención de quedarse embarazada o si se encuentra en período de lactancia, a menos que su médico le indique lo contrario.
Conducción y uso de máquinas
Es poco probable que beclometasona/formoterol afecte su capacidad para conducir y utilizar máquinas. Sin embargo, si experimenta efectos secundarios como mareos y/o temblores, su capacidad para conducir u operar maquinaria puede verse afectada.
Beclometasona/Formoterol Cipla contiene alcohol
Este medicamento contiene 7 mg de alcohol (etanol) en cada pulsación. La cantidad del alcohol en una pulsación en este medicamento es equivalente a menos de 1 ml de cerveza o vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningun efecto perceptible.
3. Cómo usar Beclometasona/Formoterol Cipla
Beclometasona/Formoterol Cipla es para uso inhalatorio. Este medicamento debe inhalarse a través de la boca hacia los pulmones.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Asma
Su médico le someterá a revisiones periódicas para asegurarse de que está tomando la dosis óptima de beclometasona/formoterol. Su médico siempre ajustará el tratamiento a la dosis mínima que mejor controle sus síntomas.
Este medicamento puede ser prescrito por su médico de dos maneras distintas:
- Use beclometasona/formoterol diariamente para tratar su asma junto con un inhalador “de rescate” por separado para tratar un repentino empeoramiento de los síntomas del asma, como dificultad en la respiración, pitidos y tos.
- Use beclometasona/formoterol diariamente para tratar su asma y también use beclometasona/formoterol para tratar un repentino empeoramiento de los síntomas del asma, como dificultad en la respiración, sibilancias y tos.
- Uso de Beclometasona/Formoterol Cipla junto con un inhalador “de rescate” por separado:
Adultos y pacientes de edad avanzada:
La dosis recomendada de este medicamento es de una a dos pulsaciones dos veces al día. La dosis diaria máxima es de 4 pulsaciones.
Recuerde:Debe llevar siempre consigo un inhalador “de rescate” de acción rápida para tratar un empeoramiento de síntomas de asma o un ataque repentino de asma.
- Uso de Beclometasona/Formoterol Cipla como único inhalador para el asma:
Adultos y pacientes de edad avanzada:
La dosis recomendada de este medicamento es de una pulsación por la mañana y una pulsación por la noche.
Debe también usar beclometasona/formoterol como inhalador “de rescate” para tratar síntomas repentinos de asma.
Si tiene síntomas de asma, inhale una pulsación y espere unos pocos minutos. Si no se siente mejor, inhale otra pulsación.
No inhale más de 6 pulsaciones de rescate de beclometasona/formoterol al día.
La dosis diaria máxima de beclometasona/formoterol es de 8 pulsaciones.
Si considera que necesita más pulsaciones al día para controlar los síntomas del asma, contacte con su médico para que le aconseje. Puede necesitar cambiar su tratamiento.
Uso en niños y adolescentes menores de 18 años:
Los niños y los adolescentes menores de 18 años de edad NO deben tomar este medicamento.
Enfermedad pulmonar obstructiva crónica (EPOC)
Adultos y pacientes de edad avanzada:
La dosis recomendada es de dos pulsaciones por la mañana y dos pulsaciones por la noche.
Pacientes de riesgo:
Los pacientes de edad avanzada no necesitan que se les ajuste la dosis. No se dispone de información sobre el uso de este medicamento en pacientes con problemas hepáticos o renales.
Beclometasona/Formoterol Cipla es eficaz para el tratamiento del asma a una dosis de dipropionato de beclometasona que puede ser inferior a la de otros inhaladores que contienen el mismo componente. Si previamente ha estado utilizando otro inhalador que contenía dipropionato de beclometasona, su médico le aconsejará sobre la dosis exacta de este medicamento que debe tomar para el asma.
No aumente la dosis.
Si cree que el medicamento no es muy eficaz, consulte siempre a su médico antes de aumentar la dosis.
Forma de administración
Beclometasona/Formoterol Cipla es para uso inhalatorio
Este medicamento se encuentra en un cartucho presurizado dentro de una cubierta de plástico con una boquilla. Hay un contador por la parte trasera del inhalador que indica cuantas dosis quedan. Cada vez que presiona el cartucho, una pulsación de medicamento se libera y el contador descuenta una dosis. Tenga cuidado de no dejar caer el inhalador ya que esto podría provocar que descontara el contador.
Comprobación del inhalador
Antes de usar el inhalador por primera vez, o si no lo ha usado durante 14 días o más, debe comprobar su inhalador para asegurarse de que funciona correctamente.
- Retire el capuchón protector de la boquilla.
- Mantenga el inhalador en posición vertical con la boquilla en la parte inferior.
- Dirija la boquilla lejos de usted y presione con firmeza el cartucho para liberar una pulsación.
- Compruebe el contador de dosis. Si está comprobando su inhalador por primera vez, el contadordebe indicar 120.
Cómo usar su inhalador
Cuando sea posible, póngase de pie o sentado en posición erguida para realizar la inhalación.
Antes de comenzar a inhalar, verifique el contador de dosis: cualquier número entre "1" y "120" muestra que quedan dosis. Si el contador de dosis muestra "0", no quedan dosis: deseche su inhalador y obtenga uno nuevo.
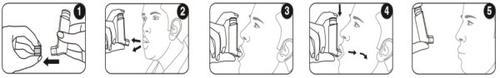
- Retire el capuchón protector de la boquilla y compruebe que esté limpia y libre de polvo y suciedad u otras partículas extrañas.
- Espire tan lenta y profundamente como sea posible.
- Mantenga el cartucho en posición vertical con el cuerpo hacia arriba y coloque la boquilla entre los labios. No muerda la boquilla.
- Inspire lenta y profundamente por la boca y, justo al empezar a inspirar, presione con firmeza la parte superior del inhalador para liberar una pulsación. Si tiene las manos débiles, puede ser más fácil sostener el inhalador con ambas manos: sostenga la parte superior del inhalador con ambos dedos índices y su parte inferior con ambos pulgares.
- Aguante la respiración tanto tiempo como pueda y, finalmente, retire el inhalador de la boca y espire lentamente. No expulse el aire en el inhalador.
En caso de que deba inhalar una nueva pulsación, mantenga el inhalador en posición vertical durante aproximadamente medio minuto y, a continuación, repita los pasos del 2 al 5.
Importante: No realice los pasos del 2 al 5 demasiado rápido.
Tras la administración, cierre con el capuchón protector y compruebe el contador de dosis.
Debería reemplazar el inhalador cuando el contador muestre el número 20. Deje de usar el inhalador cuando el contador marque 0 ya que las pulsaciones sobrantes en el dispositivo puede que no sean suficientes para tomar la dosis completa.
Si observa una “niebla” que se escapa por la parte superior del inhalador o por la comisura de los labios, significa que el medicamento no va a llegar a sus pulmones como debería. Tome otra pulsación siguiendo las instrucciones empezando de nuevo por el paso 2.
Para reducir el riesgo de infección fúngica en la boca y la garganta, enjuáguese la boca o haga gárgaras con agua o bien cepíllese los dientes cada vez que use el inhalador.
Si cree que el efecto de este medicamento es demasiado fuerte o insuficiente, consulte a su médico o farmacéutico.
Si le parece difícil pulsar el inhalador mientras inicia la respiración, puede usar el dispositivo espaciador AeroChamber Plus™. Consulte con su médico, farmacéutico o enfermero sobre el uso de este dispositivo.
Es importante que lea el prospecto provisto con el dispositivo espaciador AeroChamber Plus™ y que siga las instrucciones en cómo usar el dispositivo espaciador AeroChamber Plus™ y cómo limpiarlo, cuidadosamente.
Limpieza
Debe limpiar el inhalador una vez por semana.
Cuando lo limpie, no saque el cartucho del pulsador y no use agua ni otros líquidos para limpiar el inhalador.
Para limpiar el inhalador:
- Retire el capuchón de la boquilla separándolo del inhalador.
- Pase un trapo limpio y seco de tela o papel por el interior y exterior de la boquilla y el pulsador.
- Coloque de nuevo el capuchón de la boquilla.
Si usa más Beclometasona/Formoterol Cipla del que debe
- Si usa más formoterol del que debiera, puede experimentar los siguientes efectos adversos: náuseas, vómitos, pulso acelerado, palpitaciones, trastornos del ritmo cardíaco, ciertas alteraciones electrocardiográficas (señal cardiaca), dolor de cabeza, temblores, somnolencia, exceso de ácido en la sangre, bajos niveles de potasio en sangre y altos niveles de glucosa en sangre. Su médico puede solicitarle análisis de sangre para comprobar sus concentraciones de potasio y glucosa en sangre.
- Tomar demasiado dipropionato de beclometasona puede ocasionar alteraciones a corto plazo en el funcionamiento de las glándulas suprarrenales. Esta situación mejorará en unos días. Sin embargo, su médico puede comprobar su concentración de cortisol en suero.
Consulte a su médico si experimenta alguno de estos síntomas.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Beclometasona/Formoterol Cipla
No tome una dosis doble para compensar una dosis olvidada. Tómelo tan pronto como recuerde. Si es casi la hora de la siguiente dosis, no tome la dosis que olvido, limítese a tomar la dosis siguiente a la hora correcta.
Si interrumpe el tratamiento con Beclometasona/Formoterol Cipla
No disminuya la dosis ni deje de usar el medicamento. Aunque se sienta mejor, no deje de usar este medicamento ni disminuya la dosis. Si desea hacerlo, consulte a su médico. Es muy importante usar este medicamento de forma regular aunque no presente síntomas.
Si el asma empeora
Si los síntomas empeoran o le resulta difícil controlarlos (por ejemplo, si aumenta la frecuencia de uso del inhalador de acción rápida por separado o beclometasona/formoterol como inhalador de rescate), o bien si el inhalador de acción rápida o beclometasona/formoterol no contribuyen a mejorar los síntomas, acuda al médico de inmediato. Puede que el asma esté empeorando y que su médico tenga que aumentar la dosis de beclometasona/formoterol o prescribirle un tratamiento alternativo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Al igual que con los otros tratamientos con inhaladores, existe riesgo de empeoramiento de la dificultad para respirar y las sibilancias inmediatamente después de usar beclometasona/formoterol, lo que se conoce como broncoespasmo paradójico. Si esto sucede, SUSPENDA el uso de beclometasona/formoterol y utilice enseguida su inhalador de acción rápidapara tratar los síntomas de dificultad respiratoria y sibilancias. Póngase de inmediato en contacto con su médico.
Avise a su médico inmediatamente si experimenta reacciones de hipersensibilidad, como alergias cutáneas, picor, erupción, enrojecimiento cutáneo, hinchazón en la piel o las mucosas, especialmente en los ojos, la cara, los labios y la garganta.
Otros efectos adversos se enumeran a continuación en función de su frecuencia.
Frecuentes(afectan a menos de 1 de cada 10 personas):
- infecciones fúngicas (de la boca y garganta)
- dolor de cabeza
- ronquera
- dolor de garganta
Neumonía en pacientes con EPOC: informe a su médico si usted tiene cualquiera de los siguientes síntomas mientras inhala este medicamento, podrían ser síntomas de una infección pulmonar:
- fiebre o escalofríos
- aumento de la producción de moco, cambio en el color del moco
- aumento de la tos o aumento de dificultades para respirar
Poco frecuentes(afectan a menos de 1 de cada 100 personas):
- latido cardíaco excepcionalmente rápido y trastornos del ritmo cardíaco
- ciertas alteraciones electrocardiográficas (ECG).
- ataques de asma
- temblores
- inquietud
- mareo
- palpitaciones
- sintomas gripales
- infecciones fúngicas en la vagina
- inflamación de los senos
- inflamación del oido
- irritación de garganta
- tos y tos productiva
- náuseas
- alteraciones o disminución del sentido del gusto
- quemazón de los labios
- sequedad de boca
- dificultad para tragar
- indigestión
- malestar gástrico
- diarrea
- dolor y calambres musculares
- enrojecimiento de la cara
- exceso de sudoración
- aumento de la circulación sanguínea en ciertos tejidos del organismo,
- rinitis
Alteraciones de determinados componentes de la sangre:
- disminución del número de leucocitos
- aumento del número de plaquetas
- caida de la concentración de potasio en sangre
- aumento de la concentración de glucosa en sangre
- aumento de la concentración de insulina, ácidos grasos libres y cetonas en sangre
- erupción o ronchas
Los siguientes efectos adversos también se han recogido como “poco frecuentes” en pacientes con Enfermedad Pulmonar Obstructiva Crónica:
- reducción de la cantidad de cortisol en sangre; esto es causado por el efecto de los corticoides en su glándula adrenal
- latidos irregulares
Raros(afectan a menos de 1 de cada 1.000 personas)
- sensación de opresión en el pecho
- sensación de pérdida de latidos
- aumento o reducción de la tensión arterial
- inflamación del riñón
- hinchazón en la piel y en las mucosas persistente durante varios días
Muy raros(afectan a menos de 1 de cada 10.000 personas)
- empeoramiento del asma
- dificultad para respirar
- disminución del número de plaquetas,
- hinchazón en las manos y los pies
La inhalación de corticosteroides a dosis altas durante un período prolongado puede causar efectos sistémicos en muy raras ocasiones.Éstos incluyen:
- problemas de funcionamiento de las glándulas suprarrenales (supresión de la función suprarrenal)
- mayor presión intraocular (glaucoma)
- cataratas
- retraso del crecimiento (crecimiento lento en niños y adolescentes)
- disminución de la densidad mineral ósea (debilitamiento de los huesos)
Desconocidos (la frecuencia no puede estimarse a partir de los datos disponibles)
- trastornos del sueño
- depresión o sentirse preocupado
- inquieto
- nervioso
- sobre-excitado o irritable.
Estos efectos pueden ocurrir especialmente en niños.
- visión borrosa
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Beclometasona/Formoterol Cipla
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Antes de la dispensación: conservar en nevera (entre 2 ºC y 8ºC)
- Después de la dispensación (medicamendo dispensado por su farmacéutico):
- No utilice este medicamento tras 3 meses desde la fecha en que se lo dispensó su farmacéutico y nunca lo utilice después de la fecha de caducidad que aparece en la caja y la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
- No conservar el inhalador a temperatura superior a 25ºC
- Si el inhalador ha estado expuesto a un frío intenso, caliéntelo con sus manos durante unos minutos antes de usar. No lo caliente nunca por medios artificiales.
Advertencia: el cartucho contiene un líquido presurizado. No exponga el cartucho a temperaturas superiores a 50ºC. No perfore el cartucho.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Beclometasona/Formoterol Cipla
- Los principios activos son: dipropionato de beclometasona y fumarato de formoterol dihidrato. Cada pulsación/dosis medida del inhalador contiene 100 microgramos de dipropionato de beclometasona y 6 microgramos de fumarato de formoterol dihidrato. Esto corresponde a una dosis liberada de la boquilla de 84,6 microgramos de dipropionato de beclometasona y 5,0 microgramos de fumarato de formoterol.
- Este medicamento contiene gases fluorados de efecto invernadero.
- Cada inhalador contiene 8,15 g de HFC-134a (Norflurano) que corresponden a 0,012 toneladas de CO2 equivalente (potencial de calentamiento global PCG = 1430).
- Los demás componentes son: etanol anhidro y ácido clorhídrico.
Aspecto deBeclometasona/Formoterol Ciplay contenido del envase
Beclometasona/Formoterol Cipla está envasado en un cartucho presurizado de aluminio de 19 mL sellado con una válvula dosificadora e insertado en un pulsador de plástico de propileno con contador de dosis que incorpora una boquilla y provisto de una tapa protectora de plástico.
Tamaños de envase:
1 envase presurizado que proporciona 120 pulsaciones o
2 envases presurizados que proporcionan 120 pulsaciones cada uno
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
Cipla Europe NV
De Keyserlei 60C, Bus-1301,
2018 Amberes
Bélgica
Responsable de la fabricación:
Cipla Europe NV
De Keyserlei 60C, Bus-1301,
2018 Amberes
Bélgica
S&D Pharma CZ, spol. s.r.o.
Theodor 28, 273 08,
Pchery (instalación de Pharmos a.s.),
República Checa
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Cipla Europe NV sucursal en España
C/Guzmán el Bueno 133, Edificio Britannia
28003, Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria: Beclometason/ Formoterol Cipla 100 Mikrogramm/6 Mikrogramm pro Sprühstoß Druckgasinhalation, Lösung
Bulgaria: BIBECFO 100/6 micrograms per actuation pressurised inhalation solution
Republica Checa: Beklometason/Formoterol Cipla
Alemania: Beclometason/ Formoterol Cipla 100 Mikrogramm/6 Mikrogramm pro Sprühstoß Druckgasinhalation, Lösung
España: Beclometasona/Formoterol Cipla 100 microgramos/6 microgramos/pulsación solución para inhalación en envase a presión
Francia: BÉCLOMÉTASONE/FORMOTÉROL CIPLA 100/6 microgrammes/dose, solution pour inhalation en flacon pressurisé
Italia: Beclometasone e Formoterolo Cipla
Noruega: Beklometasondipropionat/Formoterol Cipla
Rumanía: Beclometazona/Formoterol Cipla 100/6 micrograme pe doza solutie de inhalat presurizata
Suecia: Brofobec
Eslovaquia: BIBECFO 100/6 mikrogramov na inhaláciu inhalacný roztok v tlakovom obale
Fecha de la última revisión de este prospecto:enero 2025
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es.
- País de registro
- Precio medio en farmacia31.36 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BECLOMETASONA/FORMOTEROL CIPLA 100 MICROGRAMOS/6 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESIONForma farmacéutica: INHALACIÓN PULMONAR, 200 microgramos/6 microgramos/pulsaciónPrincipio activo: formoterol and beclometasoneFabricante: Cipla Europe N.V.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 100 microgramos/6 microgramos/pulsaciónPrincipio activo: formoterol and beclometasoneFabricante: Lupin Europe GmbhRequiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 100 microgramos/6 microgramosPrincipio activo: formoterol and beclometasoneFabricante: Chiesi España S.A.U.Requiere receta
Médicos online para BECLOMETASONA/FORMOTEROL CIPLA 100 MICROGRAMOS/6 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BECLOMETASONA/FORMOTEROL CIPLA 100 MICROGRAMOS/6 MICROGRAMOS/PULSACION SOLUCION PARA INHALACION EN ENVASE A PRESION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















