AVONEX 30 microgramos/0,5 ml SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar AVONEX 30 microgramos/0,5 ml SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
AVONEX 30 microgramos/0,5 ml solución inyectable en pluma precargada
(interferón beta-1a)
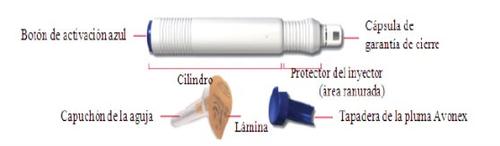
Pluma precargada
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Aunque haya utilizado Avonex con anterioridad, parte de la información puede haber cambiado.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras
personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se
trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
(Notas informativas)
Este prospecto cambia de vez en cuando.
Compruebe la posible actualización de este prospecto cada vez que renueve su receta.
(Notas informativas)
Si tiene alguna duda, consulte a su médico o farmacéutico.
Contenido del prospecto
- Qué es AVONEX y para qué se utiliza
- Qué necesita saber antes de empezar a usar AVONEX
- Cómo usar AVONEX PEN
- Posibles efectos adversos
- Conservación del AVONEX PEN
- Contenido del envase e información adicional
- Cómo inyectarse utilizando AVONEX PEN
1. Qué es AVONEX y para qué se utiliza
(Notas informativas) Avonex funciona mejor cuando lo utiliza:
No deje su tratamiento con Avonex sin hablar previamente con su médico. |
¿Qué es AVONEX?
Avonex Pen se utiliza para inyectar Avonex.El principio activo de Avonex es una proteína llamada interferón beta-1a.Los interferones son sustancias naturales elaboradas por su organismo para protegerle de infecciones y enfermedades. La proteína de Avonex se elabora exactamente con los mismos ingredientes que el interferón beta presente en el organismo humano.
¿Para qué se utiliza AVONEX?
Avonex se utiliza para tratar la esclerosis múltiple (EM).El tratamiento con Avonex puede ayudar a evitar que empeore, aunque no curará la EM.
Cada paciente tiene unos síntomas específicos de EM.Pueden ser:
- Sensación de inestabilidad o mareo, problemas para caminar, rigidez y espasmos musculares, cansancio, hormigueo en la cara, brazos o piernas
- Dolor agudo o crónico, problemas vesicales e intestinales, problemas sexuales y problemas visuales
- Dificultad para pensar y concentrarse, depresión.
La EM también tiende a exacerbarse cada cierto tiempo: esto es lo que se conoce como recidiva.
Avonex puede ayudar a reducir el número de recidivas que pueda presentar y a frenar los efectos incapacitantes de la EM.Su médico le explicará durante cuánto tiempo puede utilizar Avonex o cuándo interrumpir el tratamiento.
¿Cómo actúa AVONEX?
La esclerosis múltiple se asocia a una lesión neural (del cerebro o de la médula espinal). En la EM, el sistema de defensa de su organismo reacciona frente a su propia mielina, es decir, el aislamiento que rodea las fibras nerviosas. Cuando se lesiona la mielina, se alteran los mensajes entre el cerebro y las demás partes del organismo. Esto es lo que provoca los síntomas de la EM. Avonex parece que actúa impidiendo que el sistema de defensa de su organismo ataque la mielina.
2. Qué necesita saber antes de empezar a usar AVONEX
(Notas informativas) Avonex y las reacciones alérgicas Como Avonex está elaborado con una proteína, existe una pequeña probabilidad de que aparezca una reacción alérgica. Información adicional sobre la depresión No debe utilizar Avonex si padece una depresión severa o tiene pensamientos suicidas. Si tiene depresión, su médico puede aún prescribirle Avonex, pero es importante que le haga saber si ha tenido depresión u otro problema similar que afecte a su estado de ánimo. |
No use AVONEX
- si es alérgicoal interferón beta o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si padece una depresión graveo tiene ideas de suicidio.
Hable inmediatamente con su médico en cualquiera de estos casos.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Avonex si tiene o ha tenido en el pasado:
- Depresióno problemas que afecten a su estado de ánimo
- Pensamientos suicidas.
Debe informar inmediatamente a su médico de los cambios en el estado de ánimo, ideas de suicidio, sensación inusual de tristeza, ansiedad o desesperanza.
- Epilepsiau otros trastornos convulsivos no controlados con medicación
- Problemas renales o hepáticos graves
- Recuentos bajos de leucocitos o plaquetas,lo que puede incrementar el riesgo de infección, hemorragia o anemia
- Problemas de corazónque puedan producir síntomas como dolor torácico (angina),especialmente después de cualquier actividad; hinchazón de tobillos, dificultad respiratoria (insuficiencia cardíaca congestiva)o un ritmo cardíaco irregular (arritmias).
- Irritación en el lugar de inyección que puede producir daños en la piel y en los tejidos (necrosis en el lugar de inyección). Cuando esté listo para administrarse la inyección, siga cuidadosamente las instrucciones de la sección 7 “Cómo inyectar AVONEX PEN” al final de este prospecto. De este modo se reduce el riesgo de reacciones en el lugar de inyección.
Hable con su médico si presenta alguna de estas enfermedadeso si empeoran mientras usa Avonex.
Durante el tratamiento, se pueden formar coágulos de sangre en los vasos sanguíneos pequeños . Estos coágulos podrían afectar a sus riñones. Esto puede ocurrir tras varias semanas o varios años después de comenzar el tratamiento con Avonex.
Su médico posiblemente quiera realizar controles de su tensión arterial, sangre (recuento de plaquetas) y función renal.
Informe a su médico de que está utilizando Avonex:
- Si se le va a realizar un análisis de sangre.Avonex puede interferir en los resultados.
(Notas informativas) En alguna ocasión será necesario que recuerde a otros profesionales médicos que está siendo tratado con Avonex.Por ejemplo, si se le prescriben otros medicamentos o si se le realiza un análisis de sangre, Avonex puede interaccionar con otros fármacos o con el resultado de la prueba. |
Población pediátrica
No se recomienda el uso de Avonex en niños y adolescentes ya que los datos sobre el uso de Avonex en esta población son limitados. Avonex no se debe utilizar en niños menores de 10 años de edad porque todavía no se ha establecido si funcionaría para ellos y si sería seguro.
Otros medicamentos y AVONEX
Informe a su médicosi está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, especialmente para el tratamiento de la epilepsia o de la depresión. Avonex puede afectar a otros medicamentos o resultar afectado por éstos. Esto incluye también cualquier medicamento obtenido sin receta.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se esperan efectos dañinos en el lactante. Avonex se puede utilizar durante la lactancia.
Conducción y uso de máquinas
Si tiene sensación de mareo, no conduzca.Avonex hace que algunas personas se sientan mareadas. Si le ocurre esto o si presenta cualquier otro efecto secundario que pueda afectar a su capacidad, no conduzca ni utilice máquinas.
Información importante sobre algunos de los componentes de Avonex:
Este medicamento está esencialmente exento de sodio. Contiene menos de 23 mg (1 mmol) de sodio por cada dosis semanal.
3. Cómo usar AVONEX PEN
(Notas informativas) Hay más información sobre cómo inyectarse utilizando Avonex Pen en la parte posterior de este prospecto. |
Dosis semanal recomendada
Una inyección utilizando Avonex Pen, una vez por semana.
Intente utilizar Avonex a la misma hora el mismo día de cada semana.
Autoinyección
Puede autoinyectarse utilizando Avonex Pen sin la ayuda de su médico si le han enseñado a hacerlo. Consulte las instrucciones de autoinyección al final de este prospecto (ver sección 7, Cómo inyectarseutilizando AVONEX PEN).
Si tiene problemaspara manipular Avonex Pen, consulte a su médico quien podrá ayudarle.
Duración del tratamiento con AVONEX
Su médico le informará durante cuánto tiempo debe utilizar Avonex. Es importante que utilice Avonex de forma regular. No haga ningún cambio que no le haya indicado su médico.
Si se inyecta más AVONEX del que debe
Debe administrarse la inyección utilizando únicamente un Avonex Pen, una vez por semana. Si ha utilizado más de un Avonex Pen en el plazo de tres días, contacte inmediatamente con su médico ofarmacéutico para que le aconsejen.
Si olvida una inyección
Si olvida su dosis semanal habitual,inyecte una dosis tan pronto como sea posible. A continuación deje pasar una semana antes de volver a utilizar Avonex Pen. Continúe la inyección ese día de cada semana. Si tiene un día preferido para utilizar Avonex, hable con su médico para ajustar la dosis y recuperar su día preferido. No se administre dos inyecciones para compensar la inyección olvidada.
4. Posibles efectos adversos
(Notas informativas) Aunque la lista de posibles efectos adversos puede parecer preocupante, cabe la posibilidad de que no presente ninguno de ellos. |
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves: solicite ayuda médica.
Reacciones alérgicas graves
Si presenta alguno de los síntomas siguientes:
- Hinchazón de cara, labios o lengua
- Dificultad para respirar
- Erupción.
Llame a un médico inmediatamente.No utilice Avonex hasta que haya hablado con un médico.
Depresión
Si presenta algún síntoma de depresión:
- Sensación inusual de tristeza, ansiedad o desesperanza.
Llame a un médico inmediatamente.
Problemas hepáticos
Si presenta alguno de los síntomas siguientes:
- Coloración amarillenta de la piel o de la parte blanca de los ojos (ictericia)
- Picor generalizado
- Sensación de malestar (náuseas y vómitos)
- Hematomas que aparecen fácilmente en la piel.
Llame a un médico inmediatamenteya que pueden ser manifestaciones de un posible problema hepático.
Efectos adversos observados en ensayos clínicos.
(Notas informativas) Efectos adversos observados en ensayos clínicos. Son efectos adversos notificados por personas durante la evaluación de Avonex. Las cifras se basan en el número de personas que los presentaron. Dan una idea de la probabilidad de que usted desarrolle efectos adversos similares |
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- síntomas seudogripales: dolor de cabeza, dolores musculares, escalofríos o fiebre: véase Síntomas seudogripales, más adelante
- Dolor de cabeza.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Pérdida del apetito
- Sensación de debilidad y cansancio
- Dificultad para dormir
- Depresión
- Rubor facial
- Destilación nasal
- Diarrea (deposiciones blandas)
- Sensación de malestar (náuseas o vómitos)
- Entumecimiento u hormigueo de la piel
- Erupción, hematomas cutáneos
- Aumento de la sudoración, sudores nocturnos
- Dolor en músculos, articulaciones, brazos, piernas o cuello
- Calambres musculares, rigidez en músculos y articulaciones
- Dolor, hematoma y enrojecimiento en el sitio de inyección
- Cambios en los análisis de sangre. Los síntomas que podría presentar son cansancio, infección repetida, hematoma o hemorragia sin causa aparente.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- Pérdida de cabello
- Cambios en la menstruación
- Sensación de quemazón en el sitio de inyección.
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas)
- Dificultad para respirar
- Problemas renales que incluyen cicatrización que puede reducir su función renal
Si presenta alguno o todos de los síntomas siguientes:
- Orina con espuma
- Fatiga
- Hinchazón, especialmente de tobillos y párpados, y aumento de peso.
Informe a su médico ya que pueden ser manifestaciones de un posible problema renal.
- Coágulos de sangre en los vasos sanguíneos pequeños que pueden afectar a sus riñones (púrpura trombótica trombocitopénica o síndrome urémico hemolítico). Los síntomas pueden incluir un aumento de moratones, sangrado, fiebre, debilidad extrema, dolor de cabeza, mareos o aturdimiento. Su médico puede encontrar alteraciones en su sangre y en la función renal.
Si le preocupa alguno de los efectos, hable con su médico.
Otros efectos adversos
(Notas informativas) Estos efectos se han observado en personas que utilizan Avonex pero desconocemos la frecuencia con la que aparecen. Si se siente mareado, no conduzca. |
- Déficit o exceso de actividad del tiroides
- Nerviosismo o ansiedad, inestabilidad emocional, pensamientos irracionales o alucinaciones (ver o escuchar cosas que no son reales), confusión o suicidio
- Entumecimiento, mareo, convulsiones o crisis epilépticas y migrañas
- Percepción de su latido cardíaco (palpitaciones), frecuencia cardíaca rápida o irregular, o problemas de corazón que se acompañan de los siguientes síntomas: capacidad reducida para el ejercicio, incapacidad para permanecer tumbado, dificultad respiratoria o hinchazón de tobillos
- Problemas hepáticos como los descritos previamente
- Urticaria o erupción seudovesiculosa, picor, empeoramiento de una psoriasis que ya tuviera
- Hinchazón o hemorragia en el sitio de inyección, destrucción del tejido (necrosis) o dolor torácico después de una inyección
- Aumento o pérdida de peso
- Cambios en los resultados de los análisis, como la variación de las pruebas de función hepática
- Hipertensión arterial pulmonar: Enfermedad en la que se produce un gran estrechamiento de los vasos sanguíneos de los pulmones que provoca un aumento de la presión en los vasos sanguíneos que transportan la sangre del corazón a los pulmones. La hipertensión arterial pulmonar se observó en distintos momentos, incluso varios años después del inicio del tratamiento con medicamentos que contienen interferón beta.
Si le preocupa alguno de los efectos, hable con su médico.
Efectos de la inyección
- Sensación de desmayo:La primera inyección de Avonex puede ser administrada por su médico. Es posible que se maree. Puede que incluso se desmaye. Es improbable que ocurra de nuevo.
- Inmediatamente después de la inyección puede notar los músculos tensos o muy débiles,como si se fuera a repetir la experiencia. Es raro. Solo ocurre cuando se autoinyecta y los efectos pasan rápidamente. Pueden manifestarse en cualquier momento después de comenzar el tratamiento con Avonex.
- Si nota alguna irritación en la piel u otro problema cutáneodespués de una inyección, hable con su médico.
Síntomas seudogripales
(Notas informativas) Tres formas sencillas para reducir el impacto de los síntomas seudogripales:
|
Algunas personas refieren que después de usar Avonex Pen se sienten como si tuvieran gripe.
Los signos son:
- Dolor de cabeza
- Dolores musculares
- Escalofríos o fiebre.
Estos síntomas realmente no corresponden a la gripe
No los puede transmitir a nadie. Son más frecuentes cuando utiliza Avonex por primera vez. Los síntomas seudogripales disminuyen gradualmente a medida que se administran más inyecciones.
Niños (10años de edad o mayores) y adolescentes
En los ensayos clínicos, se notificaron algunos efectos adversos con mayor frecuencia en niños y adolescentes que en adultos, p. ej., dolor muscular, dolor en una extremidad, fatiga y dolor articular.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
Con objeto de mejorar la trazabilidad de este medicamento, su médico o farmacéutico debe registrar el nombre y el número de lote del medicamento que se le ha administrado en su historia clínica. También puede que usted desee tomar nota de esta información por si se le pide en el futuro.
5. Conservación de del AVONEX PEN
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta.
Avonex Pen contiene una jeringa precargada de Avonex. Conservar en el embalaje original para
protegerlo de la luz.
Conservar en nevera (entre 2ºC y 8ºC). No congelar.
Avonex Pen puede también conservarse a temperatura ambiente (entre 15ºC y 30ºC) durante un
semana como máximo.
No utilice Avonex Pen si observa que:
- La pluma está rota.
- La solución tiene color y puede ver partículas flotando.
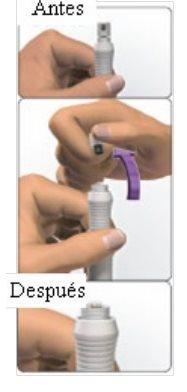
- La cápsula de garantía de cierre está rota.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de AVONEX PEN
El principio activoes: interferón beta-1a 30 microgramos/0,5 ml.
Los demás componentesson: acetato sódico trihidrato, ácido acético glacial, clorhidrato de arginina, polisorbato 20 y agua para inyectables.
Aspecto de AVONEX PEN y contenido del envase
Cada envase individual contiene un Avonex Pen, una aguja y un protector de la pluma. Avonex Pen contiene una jeringa precargada de Avonex y únicamente deberá utilizarse después de haber recibido la formación adecuada. Avonex Pen se presenta en envases con cuatro o doce, para un mes o tres meses de inyección.
Titular de la autorización de comercialización y responsable de la fabricación
El titular de la autorización de comercialización es:
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Avonex es elaborado por:
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1,
DK-3400 Hillerød,
Dinamarca
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Contacte con los representantes locales si desea una versión de este prospecto con letra más grande.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Biogen Belgium NV/SA Tél: +32 2 2191218 | Lietuva Biogen Lithuania UAB Tel: +370 5 259 6176 |
| Luxembourg/Luxemburg Biogen Belgium NV/SA Tél: +32 2 2191218 |
Ceská republika Biogen (Czech Republic) s.r.o. Tel: +420 255 706 200 | Magyarország Biogen Hungary Kft. Tel.: +36 1 899 9883 |
Danmark Biogen Denmark A/S Tlf.: +45 77 41 57 57 | Malta Pharma. MT Ltd.. Tel: +356 21337008 |
Deutschland Biogen GmbH Tel: +49 (0) 89 99 6170 | Nederland Biogen Netherlands B.V. Tel: +31 20 542 2000 |
Eesti Biogen Estonia OÜ Tel: +372 618 9551 | Norge Biogen Norway AS Tlf: +47 23 40 01 00 |
Ελλ?δα Genesis Pharma SA Τηλ: +30 210 8771500 | Österreich Biogen Austria GmbH Tel: +43 1 484 46 13 |
España Biogen Spain S.L. Tel: +34 91 310 7110 | Polska Biogen Poland Sp. z o.o. Tel: +48 22 351 51 00 |
France Biogen France SAS Tél: +33 (0)1 41 37 9595 | Portugal Biogen Portugal Sociedade Farmacêutica, Unipessoal Lda. Tel: +351 21 318 8450 |
Hrvatska Biogen Pharma d.o.o. Tel: +385 1 775 73 22 | România Johnson & Johnson Romania S.R.L. Tel: +40 21 207 18 00 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenija Biogen Pharma d.o.o. Tel: +386 1 511 02 90 |
Ísland Icepharma hf Sími: +354 540 8000 | Slovenská republika Biogen Slovakia s.r.o. Tel: +421 2 323 34008 |
Italia Biogen Italia s.r.l. Tel: +39 02 584 9901 | Suomi/Finland Biogen Finland Oy Puh/Tel: +358 207 401 200 |
Κ?προς Genesis Pharma Cyprus Ltd Τηλ: +357 22 76 57 15 | Sverige Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvija Biogen Latvia SIA Tel: +371 68 688 158 |
Fecha de la última revisión de este prospecto:08/2024.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea/Espacio Económico Europeo.
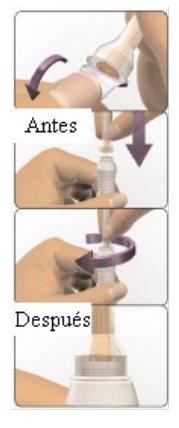
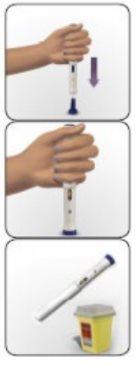
- Cómo inyectarse utilizando AVONEX PEN
Avonex Pen (un solo uso)
Contenido del envase – Avonex Pen, aguja y protector de Avonex Pen
|
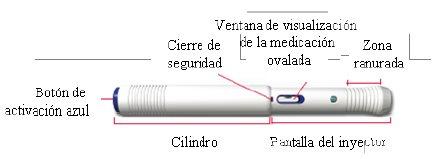
| 4 Compruebe que la pantalla del inyector esté correctamente extendida
|
| 5 Compruebe el líquido
Si la solución está turbia, tiene color o contiene partículas flotando, no utilice esta pluma. Las burbujas de aire son normales. |
| |
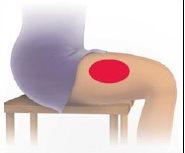
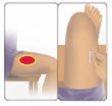
| 1 Limpie el sitio de inyección Si necesita, utilice una toallita empapada con alcohol para limpiar la piel en el lugar de inyección elegido. Deje secar la piel. Consejo: La mejor zona es la parte superior externa del muslo. |
| 2 Ponga Avonex Pen sobre la piel
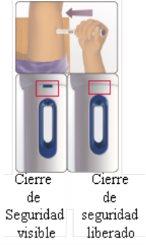
Consejo: Tenga cuidado de no pulsar el botón de activación azul demasiado pronto.
Consejo: Continúe sujetando la pluma con firmeza sobre la piel. |
|
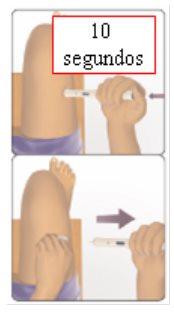
Escuchará un “clic” que indica que el proceso de inyección ha comenzado. No aparte la pluma de la piel.
|
|
inyección
|
| 5 Eliminación
Consejo: No sujete el protector de la pluma.Podrá sufrir una lesión por pinchazo.
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a AVONEX 30 microgramos/0,5 ml SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 30 µgPrincipio activo: Interferon beta-1aFabricante: Biogen Netherlands B.V.Requiere recetaForma farmacéutica: INYECTABLE, 22 µgPrincipio activo: Interferon beta-1aFabricante: Merck Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 44 µgPrincipio activo: Interferon beta-1aFabricante: Merck Europe B.V.Requiere receta
Médicos online para AVONEX 30 microgramos/0,5 ml SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de AVONEX 30 microgramos/0,5 ml SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes